K2010
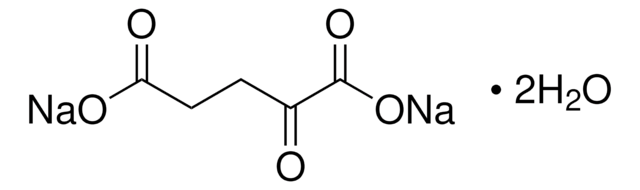
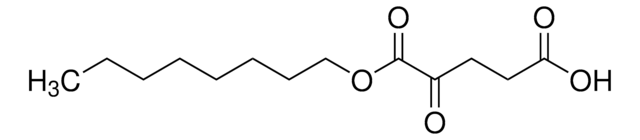
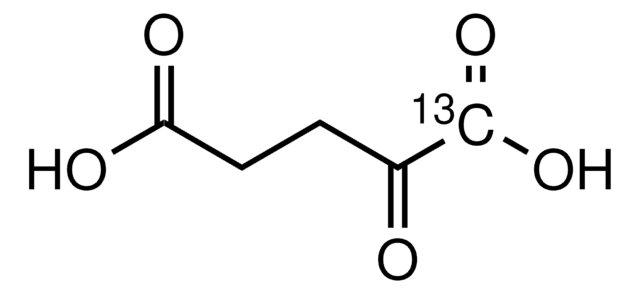
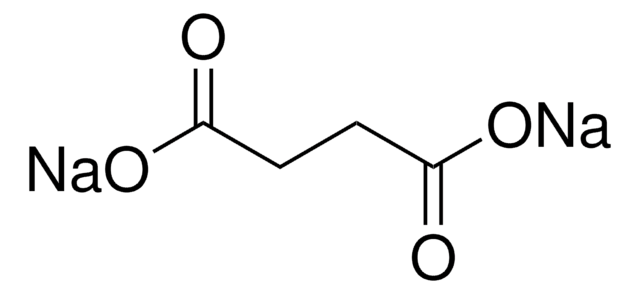
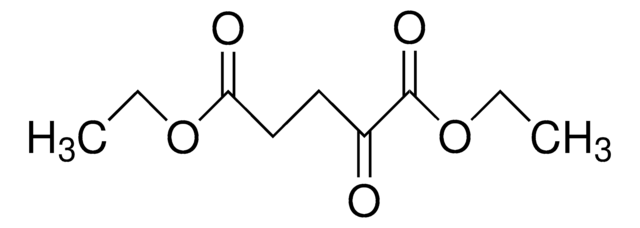
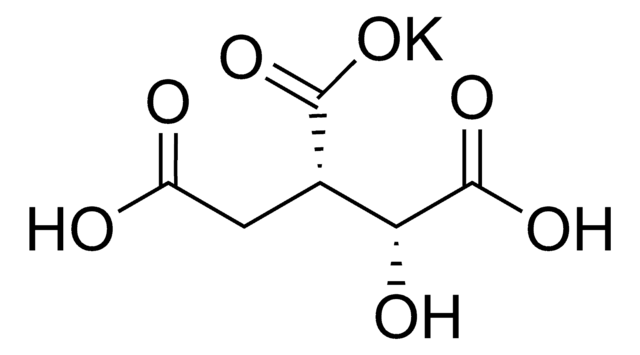
α-Ketoglutaric acid sodium salt
BioUltra
Synonym(s):
2-Oxoglutaric acid monosodium salt, 2-Oxopentanedioic acid monosodium salt, Sodium 2-oxoglutarate monobasic
About This Item
Recommended Products
product line
BioUltra
Quality Level
Assay
≥98% (perchloric acid titration)
form
powder
impurities
≤0.0005% Phosphorus (P)
≤0.1% Insoluble matter
solubility
H2O: 0.5 M, clear, colorless
anion traces
chloride (Cl-): ≤0.05%
sulfate (SO42-): ≤0.05%
cation traces
Al: ≤0.0005%
Ca: ≤0.001%
Cu: ≤0.0005%
Fe: ≤0.0005%
K: ≤0.005%
Mg: ≤0.0005%
NH4+: ≤0.05%
Pb: ≤0.001%
Zn: ≤0.0005%
storage temp.
2-8°C
SMILES string
[Na+].OC(=O)CCC(=O)C([O-])=O
InChI
1S/C5H6O5.Na/c6-3(5(9)10)1-2-4(7)8;/h1-2H2,(H,7,8)(H,9,10);/q;+1/p-1
InChI key
MOTOGHHLNTXPTI-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Application
Biochem/physiol Actions
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Sigma-Aldrich presents an article about how proliferatively active cells require both a source of carbon and of nitrogen for the synthesis of macromolecules. Although a large proportion of tumor cells utilize aerobic glycolysis and shunt metabolites away from mitochondrial oxidative phosphorylation, many tumor cells exhibit increased mitochondrial activity.
Information on fatty acid synthesis and metabolism in cancer cells. Learn how proliferatively active cells require fatty acids for functions such as membrane generation, protein modification, and bioenergetic requirements. These fatty acids are derived either from dietary sources or are synthesized by the cell.
Protocols
We describe here a rapid and sensitive method to separate and measure D-2-OHG and L-2-OHG enantiomers using high-resolution mass spectrometry (HRMS) detection.
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service