I8640
IgG from human serum
technical grade, ≥80% (SDS-PAGE), buffered aqueous solution
Synonym(s):
Human IgG
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
conjugate
unconjugated
Quality Level
grade
technical grade
Assay
≥80% (SDS-PAGE)
form
buffered aqueous solution
shipped in
dry ice
storage temp.
−20°C
target post-translational modification
unmodified
Looking for similar products? Visit Product Comparison Guide
General description
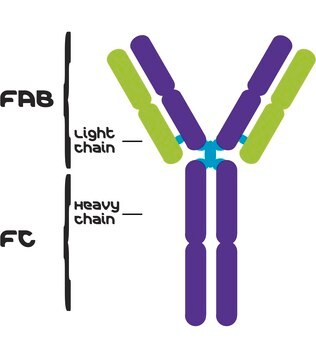
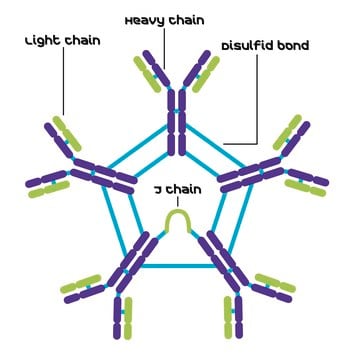
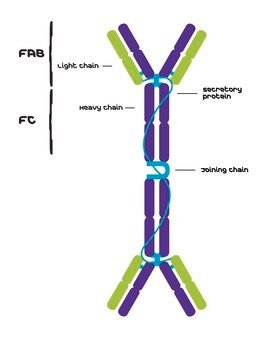
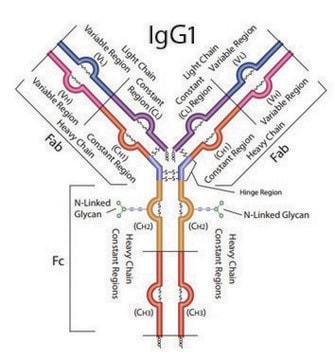
Human IgG antibodies are primarily responsible for the facilitation of humoral immune responses such as complement fixation, placental transport, and phagocytosis among many others. IgG from human serum can be used as an economical alternative for reagent grade immunoglobulins where high purity is not essential. It can also be used in immunoelectrophoresis. The product is reactive in humans.
IgG antibody subtype is the most abundant serum immunoglobulins of the immune system. It is secreted by B cells and is found in blood and extracellular fluids and provides protection from infections caused by bacteria, fungi and viruses. Maternal IgG is transferred to fetus through the placenta that is vital for immune defence of the neonate against infections
Human IgG is purified from normal human serum by fractionation.
Human IgG is purified from normal human serum by fractionation.
Application
Purified human IgG may be used as a reference antigen, standard, blocking agent, or coating protein in a variety of immunoassays including ELISA, dot immunobinding, Western immunoblotting, immunodiffusion, and immunoelectrophoresis. Other applications include starting materials for the preparation of immunogens and solid phase immunoadsorbents. Technical grade human IgG may be used as an economical alternative to the reagent grade immunoglobulins, in applications where high purity is not required. IgG from human serum was used as ELISA standard in various studies. A concentration range of 200 μg/ml to 1 mg/ml of human IgG was used for blocking in flow cytometry.
Physical form
Solution in 0.01 M phosphate buffered saline, pH 7.2, containing 15 mM sodium azide
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Gerlândia Neres Pontes et al.
FEMS immunology and medical microbiology, 47(3), 405-413 (2006-07-29)
We evaluated the ability of human maternal and cord serum antibodies to protect mice challenged with live Escherichia coli serotype O6:K2ac (E. coli O6). Mice received paired maternal or cord serum pools before a challenge with E. coli O6 to
Jaromir Ruzicka et al.
The Analyst, 131(7), 799-808 (2006-06-28)
A novel approach to real-time monitoring of protein immobilization resulted in the surprising finding that current immobilization protocols are far from optimized.
Chuan Chiang-Ni et al.
Frontiers in microbiology, 9, 2592-2592 (2018-11-15)
Group A streptococci (GAS) with spontaneous mutations in the CovR/CovS regulatory system are more invasive and related to severe manifestations. GAS can replicate inside phagocytic cells; therefore, phagocytic cells could serve as the niche to select invasive covS mutants. Nonetheless
Simone F A Wouters et al.
Bioconjugate chemistry, 31(3), 656-662 (2020-01-08)
Bioluminescent antibodies represent attractive detection agents in both bioanalytical assays and imaging. Currently, their preparation relies on genetic fusion of luciferases to antibodies or nonspecific chemical conjugation strategies. Here, we report a generic method to generate well-defined covalent antibody-luciferase conjugates
Ruben Godoy-Silva et al.
Biotechnology and bioengineering, 102(4), 1119-1130 (2008-10-30)
The effect of hydrodynamic forces on animal cell cultures, while extensively studied, still lacks significant, fundamental understanding. A previous manuscript reported on the acute exposure of CHO cells to hydrodynamic forces in a second generation convergent-divergent microfluidic device (Mollet et
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service