27645
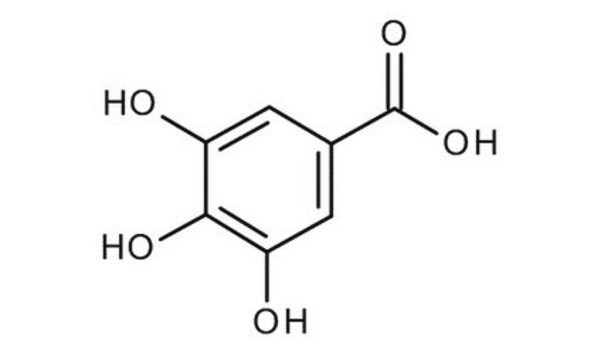
Gallic acid monohydrate
≥99% (HPLC)
Synonym(s):
3,4,5-Trihydroxybenzoic acid monohydrate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
Quality Level
Assay
≥99% (HPLC)
form
solid
impurities
7-12% water (Karl Fischer)
ign. residue
≤0.1% (as SO4)
mp
252 °C (dec.) (lit.)
anion traces
chloride (Cl-): ≤200 mg/kg
sulfate (SO42-): ≤100 mg/kg
SMILES string
OC(=O)c1cc(O)c(O)c(O)c1
InChI
1S/C7H6O5/c8-4-1-3(7(11)12)2-5(9)6(4)10/h1-2,8-10H,(H,11,12)/p-1
InChI key
LNTHITQWFMADLM-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Gallic acid monohydrate is a phenolic acid. It is a planar molecule with two intramolecular hydrogen bonds and five intermolecular hydrogen bonds.
Application
Gallic acid monohydrate can be used as a reducing agent to synthesize Tin oxide (SnO2) nanoparticles via the precipitation method using stannous chloride as a precursor salt.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
482.0 °F - closed cup
Flash Point(C)
250 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ulyana Muñoz Acuña et al.
Journal of natural products, 73(11), 1775-1779 (2010-10-30)
Two new polyisoprenylated benzophenones, 32-hydroxy-ent-guttiferone M (1) and 6-epi-guttiferone J (2), along with seven known compounds, 6-epi-clusianone (3), guttiferone A (4), xanthochymol (5), guttiferone E (6), isoxanthochymol (7), (+)-volkensiflavone (8), and (+)-morelloflavone (9), were identified from the seeds and rinds
Claudriana Locatelli et al.
Bioorganic & medicinal chemistry, 16(7), 3791-3799 (2008-02-26)
Gallic acid and gallates with the same number of hydroxyl groups and varying the length of the side carbon chain, with respective lipophilicity being defined through the ClogP values, were examined for their ability to induce apoptosis (through the DNA
Cristina Medina-Plaza et al.
Molecules (Basel, Switzerland), 24(18) (2019-09-22)
The effects of temperature and ethanol concentration on the kinetics of anthocyanin adsorption and desorption interactions with five cell wall materials (CWM) of different composition were investigated. Using temperatures of 15 °C and 30 °C and model wine with ethanol
Kristina Habschied et al.
Foods (Basel, Switzerland), 9(2) (2020-02-28)
Antioxidative molecules, such as polyphenols can preserve and prolong the freshness of packaged beers. The aim of this work was to assess the content of polyphenolic compounds (by Folin-Ciocalteu and standard European Brewery Convention method) in different types of industrially
Tarik El-Sayed Ali
European journal of medicinal chemistry, 44(11), 4385-4392 (2009-07-10)
The reaction of 5,6-diphenyl-3-hydrazino-1,2,4-triazine (1) with bis(methylthio)methylene]malononitrile (2) afforded 5-amino-1-(5,6-diphenyl-1,2,4-triazin-3-yl)-3-(methylthio)-1H-pyrazole-4-carbonitrile (3). Compound 3 reacted with thiourea to give 3,4-diaminopyrazolo[3,4-d]pyrimidine 5, which was treated with benzoyl chloride to give pyrazolo[5,4,3-kl]pyrimido[4,3-d]pyrimidine 6. Treatment of 3 with acetic anhydride produced 3-methylthio-pyrazolo[3,4-d]pyrimidine derivative 7
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service