19170
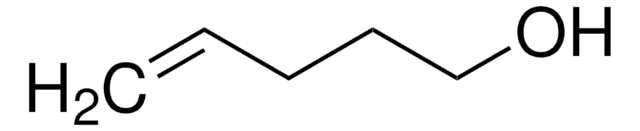
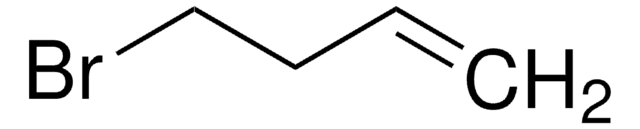
3-Buten-1-ol
purum, ≥98.0% (GC)
Synonym(s):
Allylcarbinol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH2=CHCH2CH2OH
CAS Number:
Molecular Weight:
72.11
Beilstein:
1633504
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
purum
Quality Level
Assay
≥98.0% (GC)
refractive index
n20/D 1.421 (lit.)
n20/D 1.422
bp
112-114 °C (lit.)
density
0.838 g/mL at 25 °C (lit.)
SMILES string
OCCC=C
InChI
1S/C4H8O/c1-2-3-4-5/h2,5H,1,3-4H2
InChI key
ZSPTYLOMNJNZNG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
3-Buten-1-ol was used as a starting reagent in asymmetric total synthesis of natural seimatopolide B. It was also used in the synthesis of catalytic bimetallic nanoparticles.
replaced by
Product No.
Description
Pricing
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
91.4 °F - closed cup
Flash Point(C)
33 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chada Raji Reddy et al.
Organic & biomolecular chemistry, 11(20), 3355-3364 (2013-04-09)
The asymmetric total synthesis of natural seimatopolide B along with its enantiomer is described starting from readily available 5-hexen-1-ol and 3-buten-1-ol. The key steps involved are Jacobson hydrolytic kinetic resolution, proline-catalyzed α-hydroxylation, Yamaguchi esterification and ring-closing metathesis. This asymmetric total
Alternating copolymers of functional alkenes with carbon monoxide.
Kacker S, et al.
Macromolecules, 29(18), 5852-5858 (1996)
Biologically programmed synthesis of bimetallic nanostructures.
Slocik JM and Naik RR.
Advanced Materials, 18(15), 1988-1992 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service