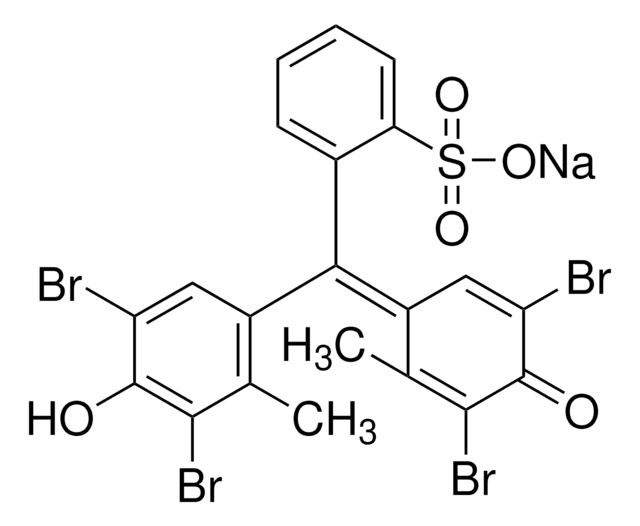
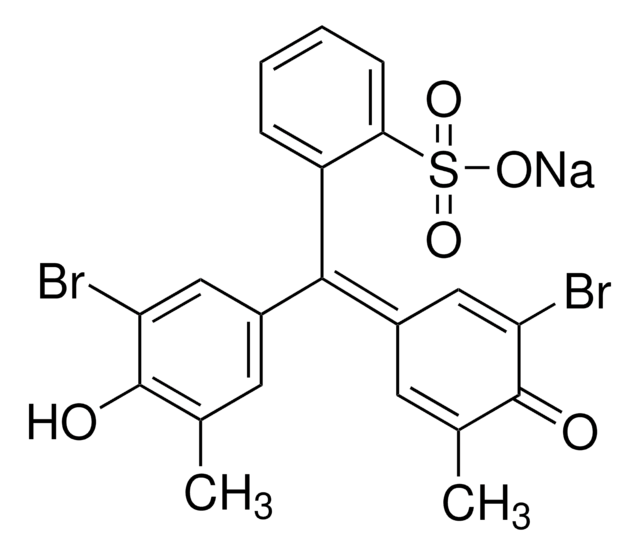
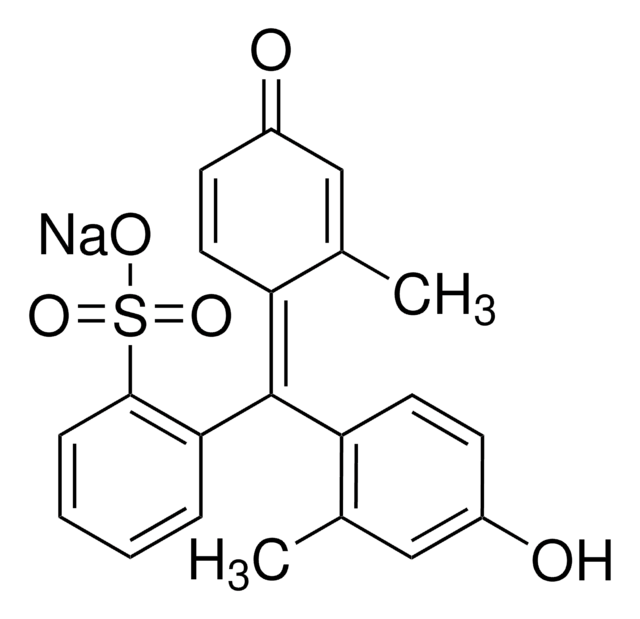
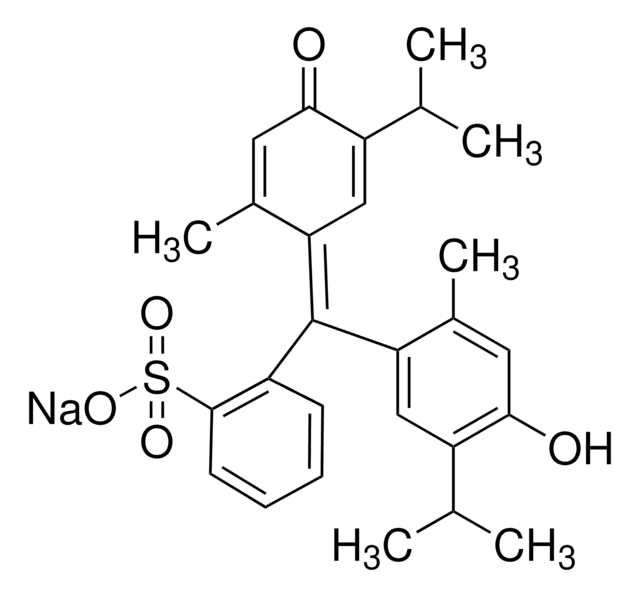
114367
Bromocresol Green sodium salt
ACS reagent, 90% (HPLC), Dye content 90 %
Synonym(s):
3′,3″,5′,5″-Tetrabromo-m-cresolsulfonphthalein sodium salt
About This Item
Recommended Products
grade
ACS reagent
Quality Level
Agency
suitable for SM 2310
suitable for SM 2320
Assay
90% (HPLC)
form
powder or crystals
composition
Dye content, 90%
visual transition interval
3.8, yellow(Visual Interval Pass)
3.8-5.2, yellow to blue
4.5, green
5.4, blue(Visual Interval Pass)
mp
230 °C (dec.) (lit.)
application(s)
diagnostic assay manufacturing
hematology
histology
storage temp.
room temp
SMILES string
[Na+].Cc1c(Br)c(O)c(Br)cc1\C(=C2\C=C(Br)C(=O)C(Br)=C2C)c3ccccc3S([O-])(=O)=O
InChI
1S/C21H14Br4O5S.Na/c1-9-12(7-14(22)19(26)17(9)24)21(13-8-15(23)20(27)18(25)10(13)2)11-5-3-4-6-16(11)31(28,29)30-21;/h3-8,26-27H,1-2H3;/q;+1/p-1
InChI key
HEFSGAHJDGZCHA-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- It is used widely as a vital stain to study blood-brain permeability.
- It is used as a tracking dye for DNA agarose gel electrophoresis, in protein determinations, and in charge-transfer complexation processes.
- It is used in TLC for the visualization of compounds with functional groups whose pKa is below 5.0.
- It has been used in the pH-sensitive colorimetric determination of glutamate decarboxylase activity.
- It has also been employed in the evaluation of paper-analytical devices for colorimetric analysis of serum samples.
- It is suitable as a selective inhibitor in kidney physiology studies.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service