SCC066
Blood-Brain Barrier hCMEC/D3 Cell Line
Human
Synonym(s):
Human BBB cell line, Human blood-brain barrier cell line, Human cerebral endothelial cell line, CMEC/D3 cell Line, hCMEC/D3 cell line
About This Item
Recommended Products
product name
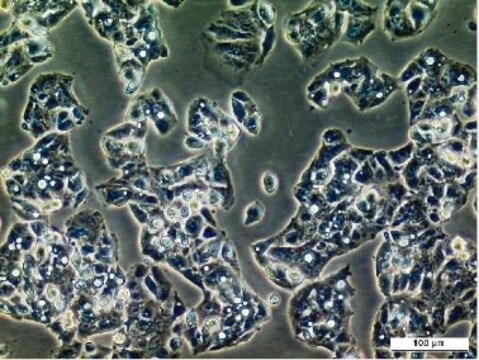
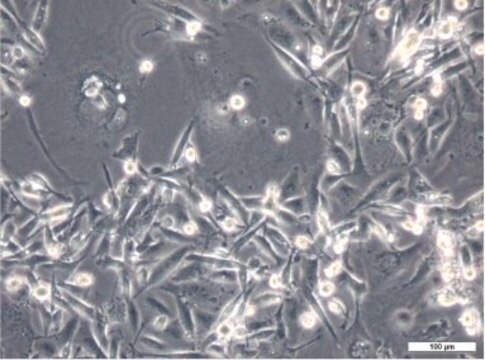
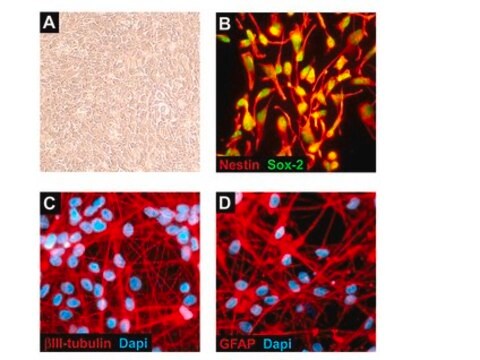
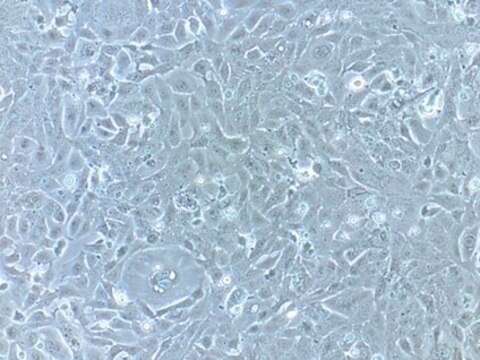
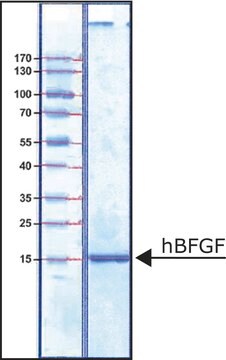
Blood-Brain Barrier hCMEC/D3 Cell Line, The hCMEC/D3 BBB cell line has been extensively characterized for brain endothelial phenotype and is a model of human blood-brain barrier (BBB) function.
biological source
human
Quality Level
technique(s)
cell culture | mammalian: suitable
drug transporter assay: suitable
shipped in
liquid nitrogen
General description
Reference:
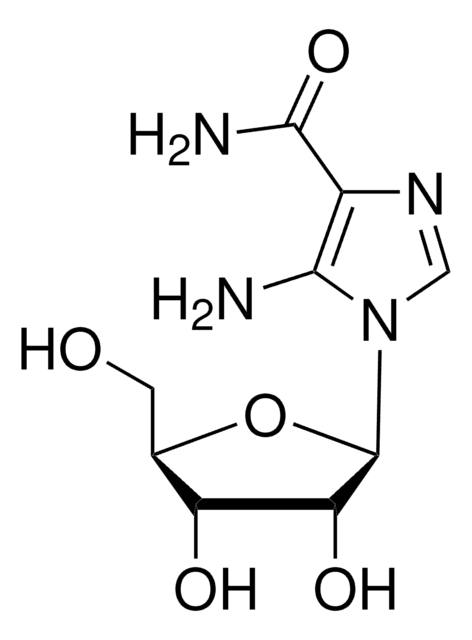
Couraud PO, et al. (2008) The human brain endothelial cell line hCMEC/D3 as a human blood‐brain barrier model for drug transport studies. Fluids Barriers CNS. 2013 Mar 26;10(1):16.
Cell Line Description
Application
Neuroscience
Neurodegenerative Diseases
Quality
• Cells are tested by PCR and are negative for Hepatitis A, B, C, HPV, Herpes and HIV-1 & 2 viruses.
• Cells are negative for mycoplasma contamination.
Storage and Stability
Disclaimer
This product contains genetically modified organisms (GMO). Within the EU GMOs are regulated by Directives 2001/18/EC and 2009/41/EC of the European Parliament and of the Council and their national implementation in the member States respectively. This legislation obliges {HCompany} to request certain information about you and the establishment where the GMOs are being handled. Click here for Enduser Declaration (EUD) Form.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service