12-219
Threonine Phosphopeptide
lyophilized powder, K-R-pT-I-R-R
Synonym(s):
Phosphopeptide K-R, Phosphopeptide K-R-pT-I-R-R, Threonine Phosphopeptide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352204
eCl@ss:
32160405
NACRES:
NA.41
Recommended Products
product name
Threonine Phosphopeptide (K-R-pT-I-R-R),
manufacturer/tradename
Upstate®
Quality Level
technique(s)
activity assay: suitable (kinase)
shipped in
wet ice
Biochem/physiol Actions
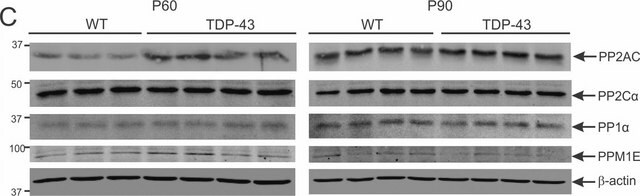
Protein Target: PP2A
Quality
Routinely evaluated by using this phosphopeptide as a substrate for PP2A (14-111) using the PP2A Immunoprecipitation Phosphatase Assay Kit (17-313). Testing may also be performed using the Ser/Thr Phosphatase Assay Kit 1 (17-127).
Physical form
Lyophilized powder
Storage and Stability
Lyophilized: Stable for 2 years at 4°C . Rehydrated: Stable for 1 year at -20°C.
Legal Information
UPSTATE is a registered trademark of Merck KGaA, Darmstadt, Germany
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
P Agostinis et al.
European journal of biochemistry, 205(1), 241-248 (1992-04-01)
p34cdc2 kinase, a critical regulator of the cell cycle, has been shown to recognize the consensus sequence S/TP in proteins such as histone H1, the retinoblastoma gene product RB and the carboxyl-terminal domain of eukaryotic RNA polymerase II. Using phosphorylated
A Donella-Deana et al.
Biochimica et biophysica acta, 1094(1), 130-133 (1991-08-13)
The four main classes of protein phosphatases (PP-1, 2A, 2B and 2C), although differing in their ability to dephosphorylate phosphopeptide substrates, invariably display a marked preference toward phosphothreonyl peptides over their phosphoseryl counterparts. Conversely, all the acidic and alkaline phosphatases
Phosphorylated synthetic peptides as tools for studying protein phosphatases.
L A Pinna et al.
Biochimica et biophysica acta, 1222(3), 415-431 (1994-07-21)
M F Santoro et al.
The Journal of biological chemistry, 273(21), 13119-13128 (1998-05-28)
Although the available evidence suggests that whereas the caspase family plays a major role in apoptosis, they are not the sole stimulators of death. A random yeast two-hybrid screen of a lymphocyte cDNA library (using caspase-3 as the bait) found
K W Harder et al.
The Biochemical journal, 298 ( Pt 2), 395-401 (1994-03-01)
The intracellular domain of human protein tyrosine phosphatase beta (HPTP beta) (44 kDa) was expressed in bacteria, purified using epitope 'tagging' immunoaffinity chromatography, and characterized with respect to kinetic profile, substrate specificity and potential modulators of enzyme activity. A chromogenic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![Tyrosine Phosphopeptide (RRLIEDAEpYAARG]](/deepweb/assets/sigmaaldrich/product/images/305/832/b833a066-1aa1-447e-8fd7-b8a873824d21/640/b833a066-1aa1-447e-8fd7-b8a873824d21.jpg)