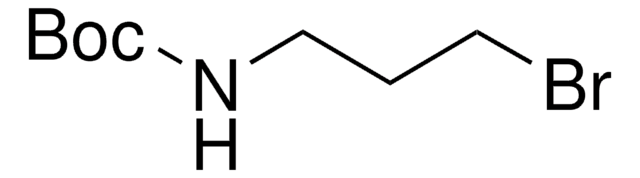
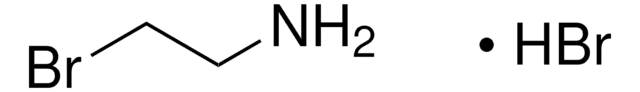
B79803
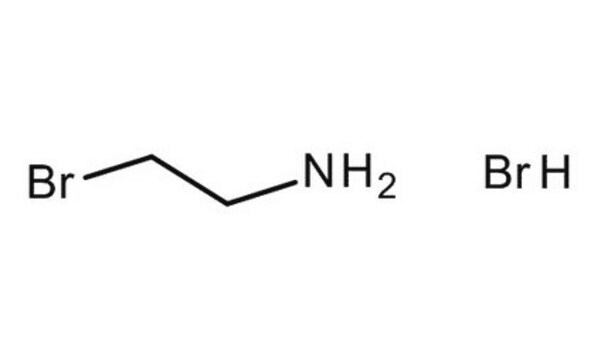
3-Bromopropylamine hydrobromide
98%
Synonym(s):
3-Aminopropyl bromide hydrobromide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
BrCH2CH2CH2NH2 · HBr
CAS Number:
Molecular Weight:
218.92
Beilstein:
3906418
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
crystals
mp
171-172 °C (lit.)
SMILES string
Br.NCCCBr
InChI
1S/C3H8BrN.BrH/c4-2-1-3-5;/h1-3,5H2;1H
InChI key
PQIYSSSTRHVOBW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
3-Bromopropylamine hydrobromide is commonly used as a reagent to introduce propylamine group to the molecular skeleton. Some of the other reported applications include:
- Synthesis of photochemically and thermally reactive azobenzene rotaxane and indolocarbazole-containing [2]rotaxane.
- Synthesis of indenoisoquinoline based active topoisomerase I inhibitors as anticancer agents.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Maya Juenet et al.
Biomaterials, 156, 204-216 (2017-12-08)
Injection of recombinant tissue plasminogen activator (rt-PA) is the standard drug treatment for thrombolysis. However, rt-PA shows risk of hemorrhages and limited efficiency even at high doses. Polysaccharide-poly(isobutylcyanoacrylate) nanoparticles functionalized with fucoidan and loaded with rt-PA were designed to accumulate
Interlocked host anion recognition by an indolocarbazole-containing [2] rotaxane.
Brown A, et al.
Journal of the American Chemical Society, 131(13), 4937-4952 (2009)
Optimization of the indenone ring of indenoisoquinoline topoisomerase I inhibitors.
Morrell A, et al.
Journal of Medicinal Chemistry, 50(18), 4388-4404 (2007)
Antagonist analogue of 6-[3 `-(1-adamantyl)-4 `-hydroxyphenyl]-2-naphthalenecarboxylic acid (AHPN) family of apoptosis inducers that effectively blocks AHPN-induced apoptosis but not cell-cycle arrest.
Dawson M I, et al.
Journal of Medicinal Chemistry, 47(14), 3518-3536 (2004)
Synthesis and anticancer activity of simplified indenoisoquinoline topoisomerase I inhibitors lacking substituents on the aromatic rings.
Nagarajan M, et al.
Journal of Medicinal Chemistry, 47(23), 5651-5661 (2004)
Global Trade Item Number
| SKU | GTIN |
|---|---|
| B79803-100G | 4061833442289 |
| B79803-25G | 4061833442296 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service