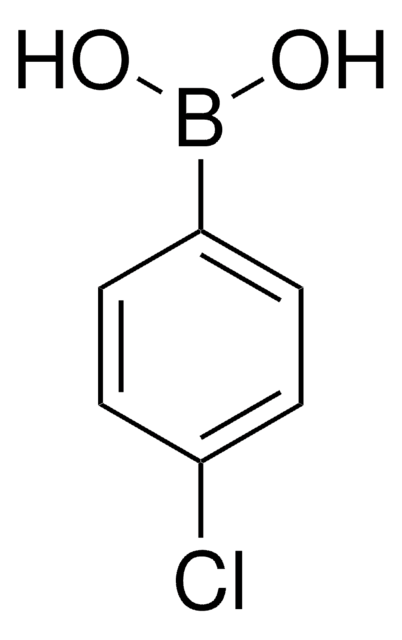
B75956
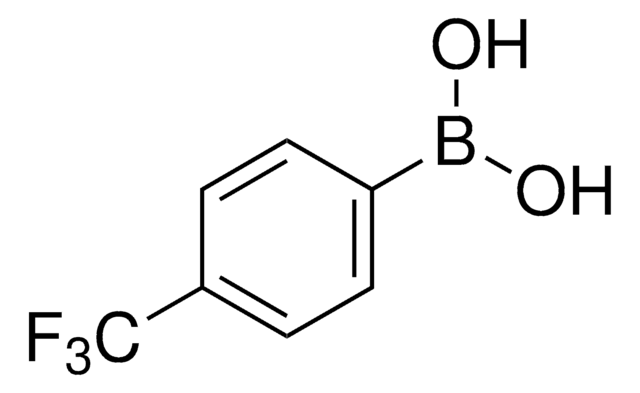
4-Bromophenylboronic acid
≥95.0%
Synonym(s):
(p-Bromophenyl)boronic acid, 4-Bromobenzeneboronic acid, 4-Bromophenylboric acid, p-Bromobenzeneboronic acid, p-Bromophenylboric acid, NSC 25407
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrC6H4B(OH)2
CAS Number:
Molecular Weight:
200.83
Beilstein:
2936347
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95.0%
95%
form
crystals
mp
284-288 °C (lit.)
SMILES string
OB(O)c1ccc(Br)cc1
InChI
1S/C6H6BBrO2/c8-6-3-1-5(2-4-6)7(9)10/h1-4,9-10H
InChI key
QBLFZIBJXUQVRF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reagent used for
Reagent used in Preparation of
- Palladium catalyzed Suzuki-Miyaura cross-couplings
- Pd(II)-catalyzed diastereoselective conjugate additions
- Palladium-catalyzed stereoselective Heck-type reaction of allylic esters with arylboronic acids
- Tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation sequence
- Copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides
- Pd-catalyzed arylative cyclization of alkyne-tethered enals or enones via carbopalladation of alkynes
- Copper-catalyzed cross-couplings
Reagent used in Preparation of
- Gallate-based obovatol analogs with potential anti-tumor activity
- Protein modulators and enzymatic and kinase inhibitors
Other Notes
Contains varying amounts of anhydride
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
James A Jordan-Hore et al.
Organic letters, 14(10), 2508-2511 (2012-05-02)
Ligand-free cationic Pd(II) catalyst with NaNO3 as an additive is a highly active catalytic system for conjugate additions to sterically hindered γ-substituted cyclohexenones. More challenging γγ- and βγ-substrates also react well to produce products with quaternary centers in good dr.
Synthesis of obovatol derivatives and their preliminary evaluation as antitumor agents
Lee, M.-S.; et al.
Bull. Korean Chem. Soc., 28, 1601-1604 (2007)
Jingyi Ye et al.
International journal of nanomedicine, 14, 5623-5636 (2019-08-24)
The objective of this study was to compare the in vitro Fick's first law, in vitro lipolysis, and in vivo rat assays for oral absorption of Biopharmaceutical Classification Systems Class II (BCS II) drugs in self-nanoemulsifying drug delivery system (SNEDDS)
Tahlia R Meola et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 129, 145-153 (2018-06-02)
The synergistic effect of nanosizing and lipid-based drug delivery systems (LBDDS) was explored to enhance formulation drug loading levels and improve drug solubilisation in the gastrointestinal environment. A novel formulation combining drug nanocrystals and silica-lipid hybrid (SLH) microparticles as a
Chuan Xiao et al.
Bioorganic & medicinal chemistry, 19(23), 7100-7110 (2011-11-01)
A series of purine nucleoside analogues bearing an aryl and hetaryl group in position 6 were prepared and their biological activities were assessed by in vitro CDK1/Cyclin B1 and CDK2/Cyclin A2 kinase assay. From the synthesized chemicals, three Xylocydine derivatives
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service