78194
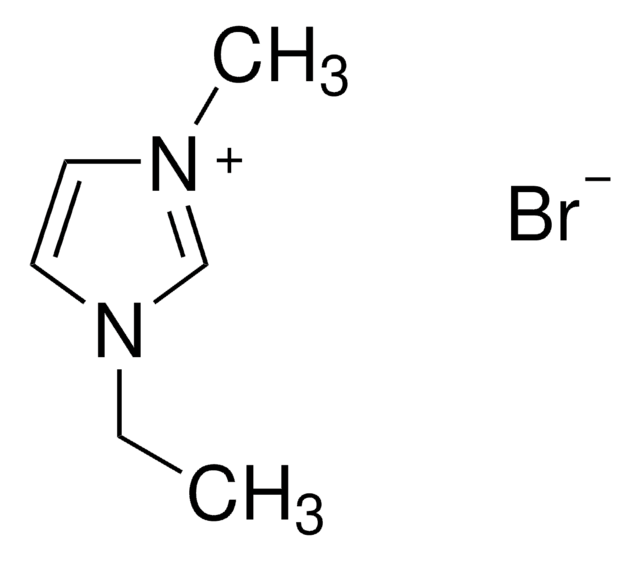
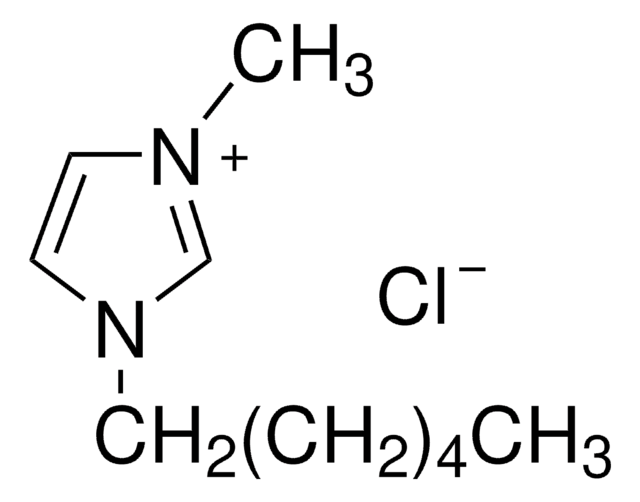
1-Butyl-2,3-dimethylimidazolium chloride
≥97.0% (HPLC/AT)
About This Item
Recommended Products
Assay
≥97.0% (HPLC/AT)
form
crystals
impurities
≤1.0% water
SMILES string
[Cl-].CCCCn1cc[n+](C)c1C
InChI
1S/C9H17N2.ClH/c1-4-5-6-11-8-7-10(3)9(11)2;/h7-8H,4-6H2,1-3H3;1H/q+1;/p-1
InChI key
HHHYPTORQNESCU-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- As a solvent in the chemical modification of polysaccharide cellulose.
- As a model ionic liquid in the conversion of a monosaccharide like fructose into 5-hydroxymethylfurfural using H2SO4.
- To prepare 1-butyl-2,3-dimethylimidazolium dicarba-7,8-nidoundecaborate by reacting with caesium dicarba-7,8-nido-undecaborate.
- To prepare mesoporous ZnAl2O4 nanomaterials, which are used as catalysts or catalyst supports.
Physical form
Other Notes
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Task-Specific Ionic Liquids (TSILs) for the selective liquid/liquid extraction of heavy metals from aqueous systems were first published by Robin D. Rogers et al. in the year 2001.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service