746991
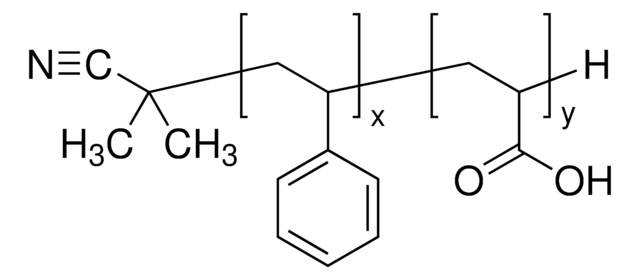
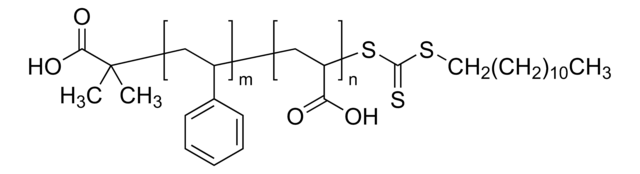
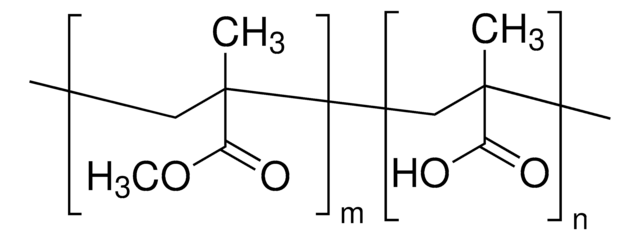
Polystyrene-block-poly(acrylic acid)
Synonym(s):
Poly(styrene)-block-poly(acrylic acid)
About This Item
Recommended Products
form
solid
mol wt
Mn 27,000-31,000 (polystyrene)
Mn 31,000-37,000 (total)
Mn 4,000-6,000 (poly(acrylic acid))
mp
258-263 °C
PDI
≤1.3
Looking for similar products? Visit Product Comparison Guide
Application
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Reversible addition–fragmentation chain transfer (RAFT) polymerization is rapidly moving to the forefront in construction of drug and gene delivery vehicles.
The development of drugs that target specific locations within the human body remains one of the greatest challenges in biomedicine today.
Over the past two decades, the rapid advance of controlled living polymerization (CLP) techniques.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service