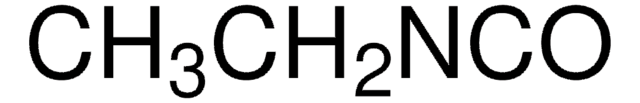66181
N-Succinimidyl N-methylcarbamate
≥97.0% (N), for peptide synthesis
Synonym(s):
MIC substitute, Methyl isocyanate substitute
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H8N2O4
CAS Number:
Molecular Weight:
172.14
Beilstein:
1531863
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
product name
N-Succinimidyl N-methylcarbamate, ≥97.0% (N)
Quality Level
Assay
≥97.0% (N)
form
solid
application(s)
peptide synthesis
functional group
amine
imide
storage temp.
2-8°C
SMILES string
CNC(=O)ON1C(=O)CCC1=O
InChI
1S/C6H8N2O4/c1-7-6(11)12-8-4(9)2-3-5(8)10/h2-3H2,1H3,(H,7,11)
InChI key
XMNGSPOWUCNRMO-UHFFFAOYSA-N
Other Notes
Safe and crystalline chemical equivalent of the toxic methyl isocyanate, MIC
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J Martinez et al.
Journal of medicinal chemistry, 25(2), 178-182 (1982-02-01)
A practical and convenient method for synthesizing antitumor compounds, N-alkyl-N-nitrosoureas, regioselectively nitrosated on the nitrogen atom bearing the alkyl group is proposed. N-Alkyl-N-nitrosocarbamates are interesting intermediates in these syntheses and yield, by reaction with amino compounds, the regioselectively nitrosated N-alkyl-N-nitrosoureas.
K. Takeda et al.
Tetrahedron Letters, 24, 4569-4569 (1983)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service