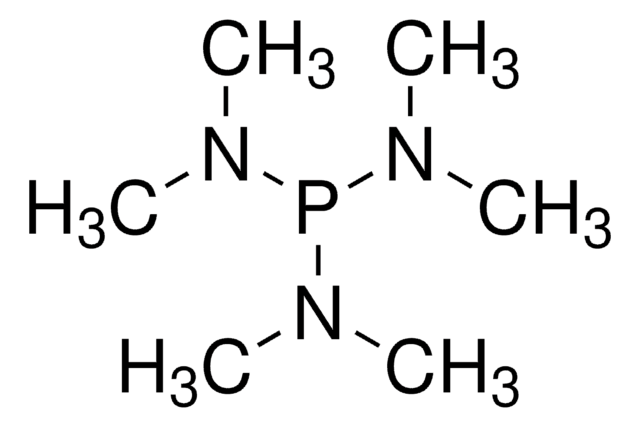
661058
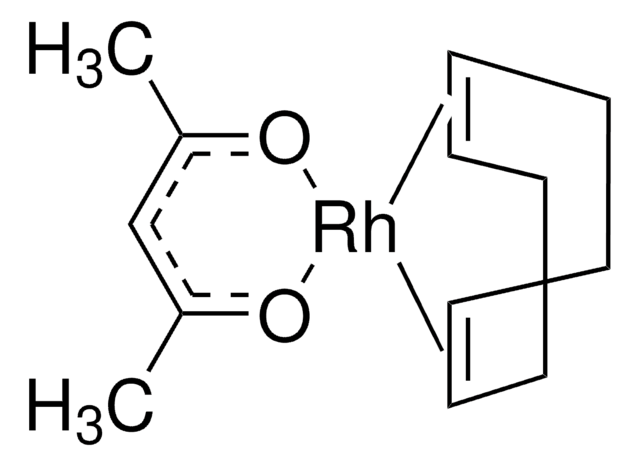
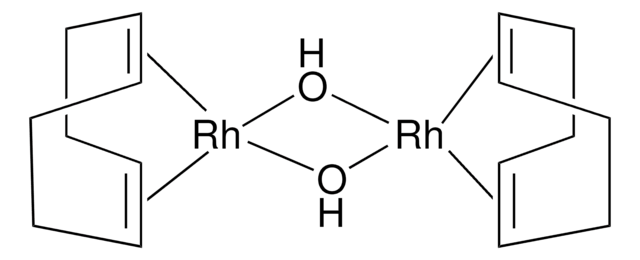
Methoxy(cyclooctadiene)rhodium(I) dimer
Synonym(s):
[Rh(OMe)(1,5-cod)]2
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C18H30O2Rh2
CAS Number:
Molecular Weight:
484.24
MDL number:
UNSPSC Code:
12161600
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
solid
Quality Level
reaction suitability
core: rhodium
reagent type: catalyst
mp
192-225 °C
storage temp.
−20°C
SMILES string
CO[Rh].CO[Rh].C1CC=CCCC=C1.C2CC=CCCC=C2
InChI
1S/2C8H12.2CH3O.2Rh/c2*1-2-4-6-8-7-5-3-1;2*1-2;;/h2*1-2,7-8H,3-6H2;2*1H3;;/q;;2*-1;2*+1/b2*2-1-,8-7-;;;;
InChI key
BDQMDBOKWREKIT-MIXQCLKLSA-N
Related Categories
Application
Precursor for organorhodium(I) species, catalyst for cascade1,4-addition-aldol reaction, 1,4-addition of alcohols, and hydroformylation
Rhodium catalyst employed in the cyclization of cyano-substituted unsaturated esters in the presence of aryl 9-BBN leading to 5- and 6-membered β-enamino esters.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Farnworth, M. V.; Cross, M. J.; Louie, J.
Tetrahedron Letters, 45, 7441-7441 (2004)
Tomoya Miura et al.
Organic letters, 9(5), 741-743 (2007-02-08)
[reaction: see text] Unsaturated esters possessing a pendent cyano moiety react with B-Ar-9-BBNs in the presence of a rhodium(I) catalyst to give the five- and six-membered beta-enamino esters in good yield. An (oxa-pi-allyl)rhodium(I) intermediate, formed by initial conjugate addition of
Kazuhiro Yoshida et al.
Journal of the American Chemical Society, 124(37), 10984-10985 (2002-09-13)
The reaction of 9-aryl-9-borabicyclo[3.3.1]nonanes (B-Ar-9BBN) with alpha,beta-unsaturated ketones and aldehydes in the presence of 3 mol % [Rh(OMe)(cod)](2) in toluene at 20 degrees C for 2 h gave high yields of the tandem 1,4-addition-aldol reaction products with high syn selectivity.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![Bicyclo[2.2.1]hepta-2,5-diene-rhodium(I) chloride dimer 96%](/deepweb/assets/sigmaaldrich/product/structures/700/585/b2e5ae1d-2b88-42c8-a071-ef828d4a104c/640/b2e5ae1d-2b88-42c8-a071-ef828d4a104c.png)