All Photos(1)
About This Item
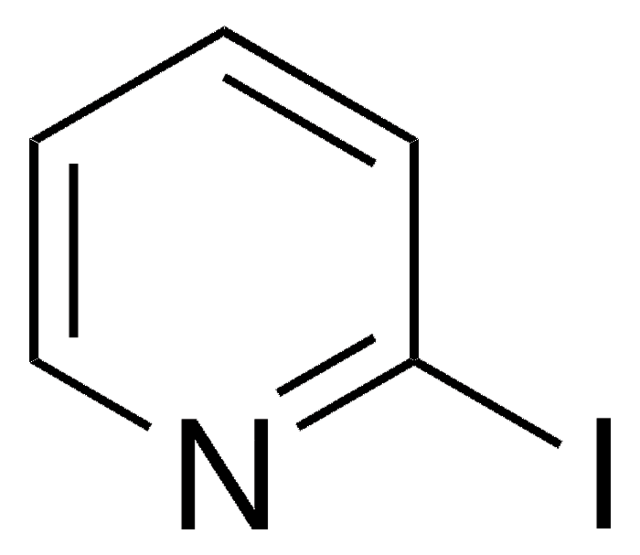
Empirical Formula (Hill Notation):
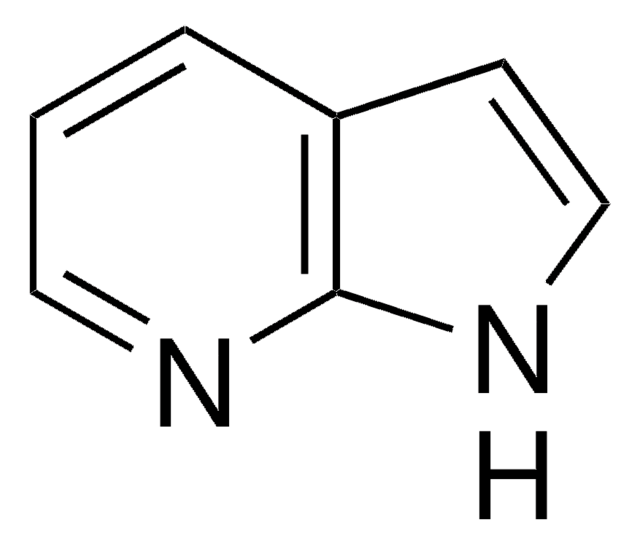
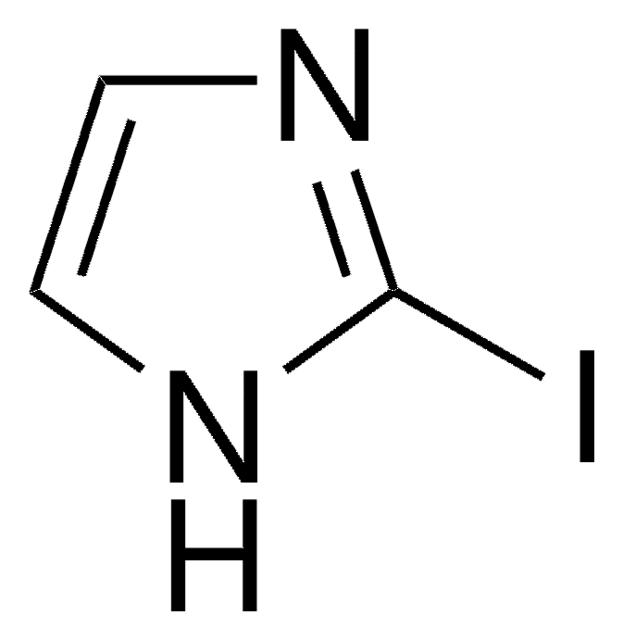
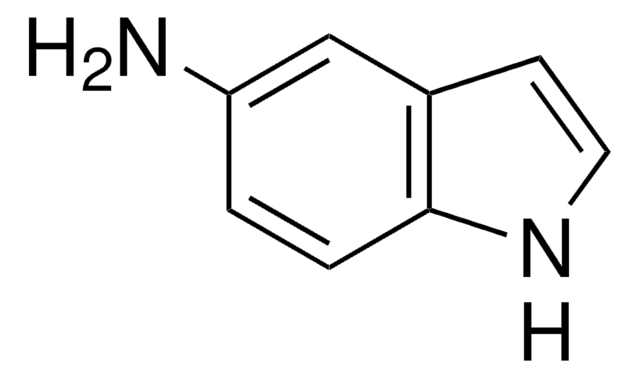
C8H6IN
CAS Number:
Molecular Weight:
243.04
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
101-104 °C (lit.)
SMILES string
Ic1ccc2[nH]ccc2c1
InChI
1S/C8H6IN/c9-7-1-2-8-6(5-7)3-4-10-8/h1-5,10H
InChI key
TVQLYTUWUQMGMP-UHFFFAOYSA-N
General description
5-Iodoindole can be synthesized via nitration of m-toluidine.
Application
5-Iodoindole (5-iodogramine) may be used in the synthesis of the following:
- 3-dimethylaminomethyl-5-iodoindole via reaction with dimethyl amine and formaldehyde
- 5-ethynyl-1H-indole obtained via refluxing with trimethylsilylacetylene in the presence of triethylamine, catalyzed by palladium and copper(I)iodide in acetonitrile
- 5-(3-hydroxyprop-1-enyl)-1H-indole via reaction with allyl alcohol in the presence of triphenyl phosphine, palladium acetate and silver acetate in dimethylformamide
- 5-(3-benzyloxyprop-1-enyl)-1H-indole via reaction with allylbenzyl ether in the presence of triphenyl phosphine, palladium acetate and silver acetate in dimethylformamide
- 5-(2-phenylethynyl)-1H-indole via refluxing with phenylacetylene catalyzed by copper(I)iodide and palladium in the presence of triethylamine in acetonitrile
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
?Convenient synthesis of 5-iodoindole?
Hydorn.EA
The Journal of Organic Chemistry, 32(12), 4100-4101 (1967)
"Synthesis and biological evaluation of new dipyrrolo [3, 4-a: 3, 4-c] carbazole-1, 3, 4, 6-tetraones, substituted with various saturated and unsaturated side chains via palladium catalyzed cross-coupling reactions"
Henon H, et al.
Bioorganic & Medicinal Chemistry, 14(11), 3825-3834 (2006)
Sooyeon Song et al.
Biotechnology and bioengineering, 116(9), 2263-2274 (2019-06-05)
The subpopulation of bacterial cells that survive myriad stress conditions (e.g., nutrient deprivation and antimicrobials) by ceasing metabolism, revive by activating ribosomes. These resuscitated cells can reconstitute infections; hence, it is imperative to discover compounds which eradicate persister cells. By
?The synthesis of 5-iodotryptophan and some derivatives"
Harvey GD
Journal of the Chemical Society, 3760-3762 (1958)
Satish Kumar Rajasekharan et al.
Pesticide biochemistry and physiology, 163, 76-83 (2020-01-25)
Multi-drug resistance in nematodes is a serious problem as lately several resistant phenotypes have emerged following the intermittent usage of synthetic nematicides. Contemporary research continues to focus on developing and/or repurposing small molecule inhibitors that are eco-friendly. Here, we describe
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service