All Photos(1)
About This Item
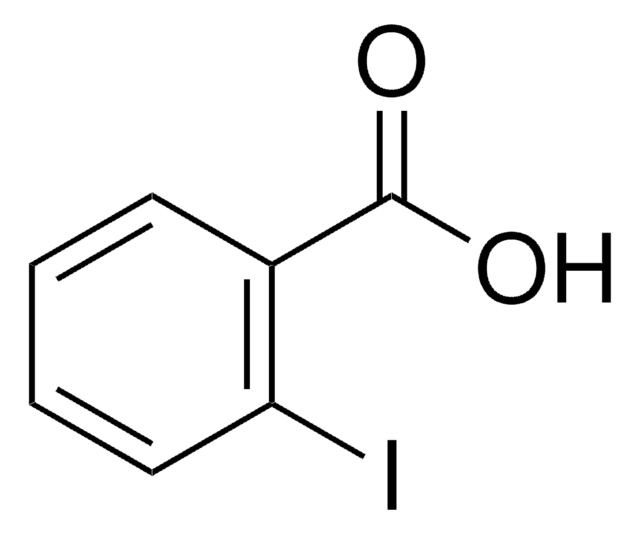
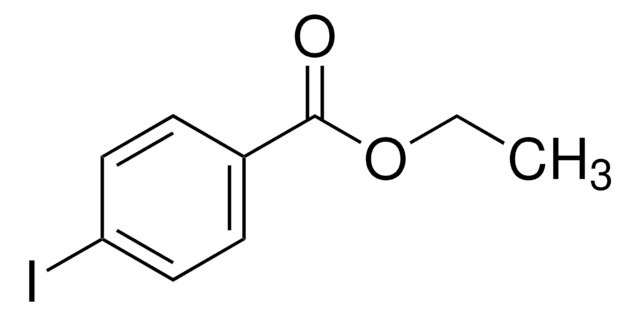
Linear Formula:
IC6H4CO2C2H5
CAS Number:
Molecular Weight:
276.07
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.581 (lit.)
bp
272 °C (lit.)
density
1.64 g/mL at 25 °C (lit.)
functional group
ester
iodo
SMILES string
CCOC(=O)c1cccc(I)c1
InChI
1S/C9H9IO2/c1-2-12-9(11)7-4-3-5-8(10)6-7/h3-6H,2H2,1H3
InChI key
POGCXCWRMMXDAQ-UHFFFAOYSA-N
General description
Ethyl 3-iodobenzoate is a halogenated aromatic ester. It affords arylzinc bromide via reaction with i-PrMgBr in THF, followed by reaction with ZnBr2.
Application
Ethyl 3-iodobenzoate may be used to synthesize:
- arylzinc bromide
- functionalized arylmagnesium compound
- ethyl3-phenylbenzoate
- ethyl 3-[(12-tert-butyldimethylsilyloxymethyl-1,12-dicarba-closo-dodecaboran)-1-yl]benzoate
- ethyl 3-(4-methoxy-1-methyl-2-oxo-1,2-dihydroquinolin-3-yl)benzoate
- ethyl 3-(1-methyl-2-oxo-4-phenyl-1,2-dihydroquinolin-3-yl)benzoate
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Nonpeptide Arginine Vasopressin Antagonists for Both V1A and V2 Receptors: Synthesis and Pharmacological Properties of 2-Phenyl-4'-((2, 3, 4, 5-tetrahydro-1H-1-benzazepin-1-yl) carbonyl) benzanilide Derivatives.
Matsuhisa A, et al.
Chemical & Pharmaceutical Bulletin, 45(11), 1870-1874 (1997)
Synthesis of 3, 4-Disubstituted Quinolin-2-(1H)-ones via Palladium-Catalyzed Decarboxylative Arylation Reactions.
Carrer A, et al.
Advanced Synthesis & Catalysis, 355(10), 2044-2054 (2013)
Shinya Fujii et al.
Bioorganic & medicinal chemistry, 17(1), 344-350 (2008-11-22)
A novel series of androgen receptor (AR) ligands bearing an acidic heterocycle with hydrogen-bonding ability as the terminal polar group was developed. Since most non-steroidal AR ligands so far known are structurally limited to nitro- or cyanobenzanilide as the polar
Copper catalyzed conjugate addition of highly functionalized arylmagnesium compounds to enones.
Varchi G, et al.
Tetrahedron, 56(18), 2727-2731 (2000)
Ni (II)-catalyzed cross-coupling between polyfunctional arylzinc derivatives and primary alkyl iodides.
Giovannini R and Knochel P.
Journal of the American Chemical Society, 120(43), 11186-11187 (1998)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service