All Photos(2)
About This Item
Empirical Formula (Hill Notation):
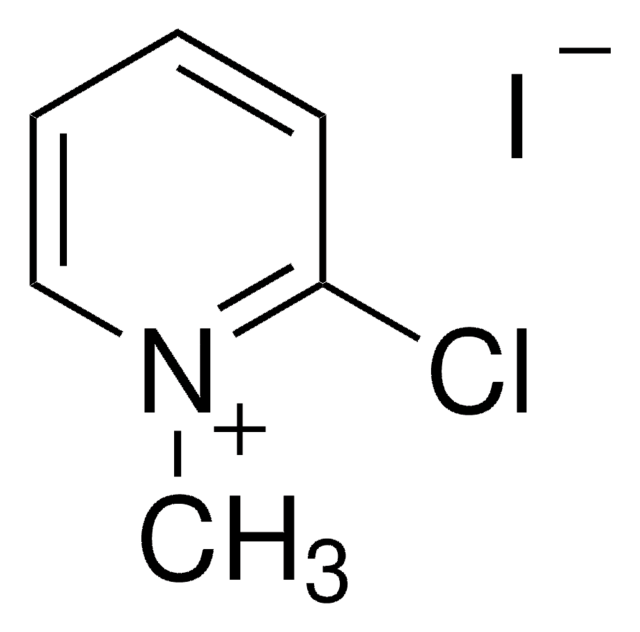
C5H7IN2
CAS Number:
Molecular Weight:
222.03
Beilstein:
3713241
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
159-161 °C (lit.)
storage temp.
2-8°C
SMILES string
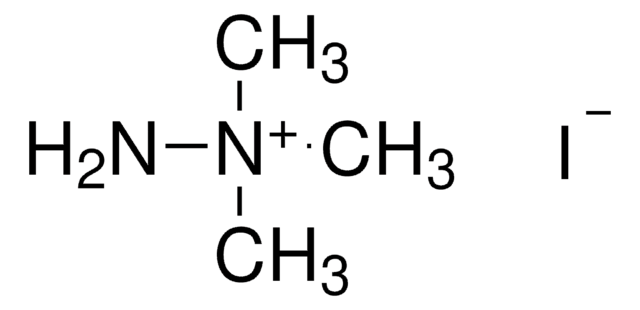
[I-].N[n+]1ccccc1
InChI
1S/C5H7N2.HI/c6-7-4-2-1-3-5-7;/h1-5H,6H2;1H/q+1;/p-1
InChI key
NDRLPYIMWROJBG-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
1-Aminopyridinium iodide, a pyridine derivative, is an important reagent for organic synthesis. It has various pharmaceutical applications. It participates in homogeneous transition metal-catalyzed reactions. Single-crystal X-ray diffraction studies suggest that the compound crystallizes in the monoclinic space group P21/c (phase II). Aza-ylide derived from 1-aminopyridinium iodide serves as a commercially available ammonia equivalent during the preparation of primary amines.
Application
1-Aminopyridinium iodide may be used in the preparation of room temperature ionic liquids (organic salts in liquid state at room temperature). It may also be used for the preparation of oligomycin A annelated with pyrazolo[1,5-a]pyridine.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Supported room temperature ionic liquid membranes for CO2/CH4 separation.
Iarikov DD, et al.
Chemical Engineering Journal, 166(1), 401-406 (2011)
Lyudmila N Lysenkova et al.
The Journal of antibiotics, 63(1), 17-22 (2009-11-17)
The first examples of chemical modification of antibiotic oligomycin A are described. The interaction of oligomycin A with hydroxylamine yielded six-membered nitrone annelated with the antibiotic at the positions 3,4,5,6,7. The reaction with 1-aminopyridinium iodide in pyridine led to pyrazolo[1,5-a]pyridine
P Andrew Evans et al.
Journal of the American Chemical Society, 131(25), 8722-8723 (2009-06-09)
The transition metal catalyzed allylic amination represents a powerful and versatile cross-coupling for the asymmetric construction of stereogenic C-N bonds that are present in secondary metabolites and medicinally important agents. We have developed a regio- and enantiospecific rhodium-catalyzed allylic amination
Crystal structure and characterization of a novel ferroelastic ionic crystal: 1-Aminopyridinium iodide (C 5 H 7 N 2)+ I-.
Owczarek M, et al.
Chemical Physics Letters, 537, 38-47 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service