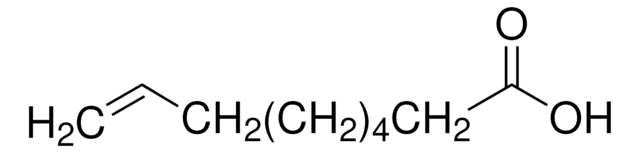All Photos(1)
About This Item
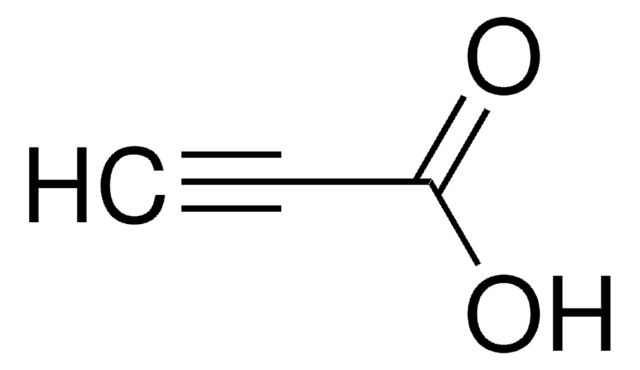
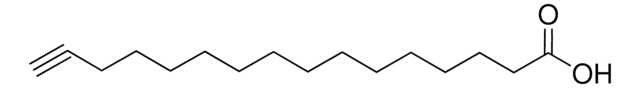
Linear Formula:
HC≡C(CH2)8CO2H
CAS Number:
Molecular Weight:
182.26
Beilstein:
1704918
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
bp
180 °C/15 mmHg (lit.)
mp
40-42 °C (lit.)
SMILES string
OC(=O)CCCCCCCCC#C
InChI
1S/C11H18O2/c1-2-3-4-5-6-7-8-9-10-11(12)13/h1H,3-10H2,(H,12,13)
InChI key
OAOUTNMJEFWJPO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
10-Undecynoic acid (10- UDYA, UDY) is an acetylenic fatty acid. It is reported as highly selective irreversible inhibitor of hepatic ω- and ω-1-lauric acid hydroxylases. Enzyme catalyzed esterification of 10-undecynoic acid has been reported. UDY has been reported to be synthesized by the dehydrobromination of 10-undecenoic acid.
Application
10-Undecynoic acid was employed as model compound to investigate the microwave assisted surface click reactions catalyzed with Cu(II)/sodium L-ascorbate.†
It may be used:
It may be used:
- As a biochemical probe in an assay for the microsomal hydroxylation of lauric acid (LA), based on HPLC with flow-through radiochemical detection.
- To form molecular layers by adsorbing on the fluorite surface.
- In the supercritical hydrothermal synthesis of iron oxide nanoparticles.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C A CaJacob et al.
Biochemistry, 25(16), 4705-4711 (1986-08-12)
The hepatic cytochrome P-450 isozymes that catalyze omega- and (omega - 1)-hydroxylation of lauric acid are specifically inactivated in vitro but not in vivo by 10-undecynoic acid. The lack of in vivo activity may result from rapid degradation of the
Gerard Lligadas et al.
Biomacromolecules, 8(6), 1858-1864 (2007-05-03)
Novel biobased aromatic triols (1,3,5-(9-hydroxynonyl)benzene and 1,3,5-(8-hydroxyoctyl)-2,4,6-octylbenzene) were synthesized through the transition-metal-catalyzed cyclotrimerization of two alkyne fatty acid methyl esters (methyl 10-undecynoate and methyl 9-octadecynoate) followed by the reduction of the ester groups to give terminal primary hydroxyl groups. A
Thiol-yne reaction of alkyne-derivatized fatty acids: biobased polyols and cytocompatibility of derived polyurethanes.
Gonzalez-Paz RJ, et al.
Polym. Chem., 3(9), 2471-2478 (2012)
Continuous hydrothermal synthesis of in situ functionalized iron oxide nanoparticles: a general strategy to produce metal oxide nanoparticles with clickable anchors.
de Tercero MD, et al.
Particle & Particle Systems Characterization, 30(3), 229-234 (2013)
C A CaJacob et al.
The Journal of biological chemistry, 263(35), 18640-18649 (1988-12-15)
Cytochrome P-450LA omega purified from clofibrate-induced rat liver oxidizes lauric acid to 11- and 12-hydroxydodecanoic acid in approximately a 1:17 ratio at a rate of 20 nmol/nmol P-450/min. In contrast, cytochrome P-450b oxidizes lauric acid much more slowly (0.5 nmol/nmol
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service