379093
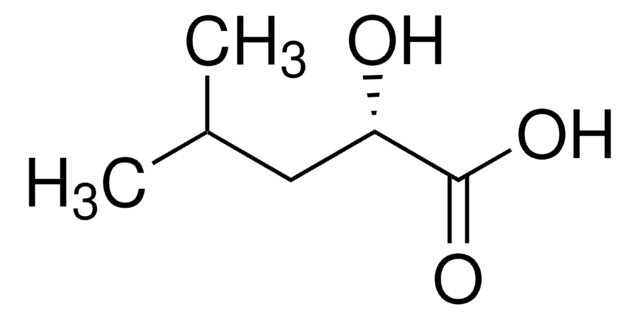
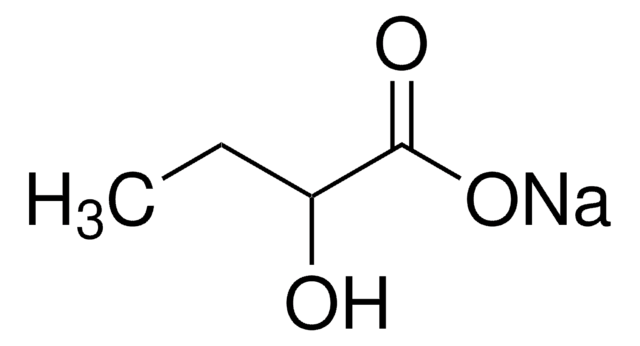
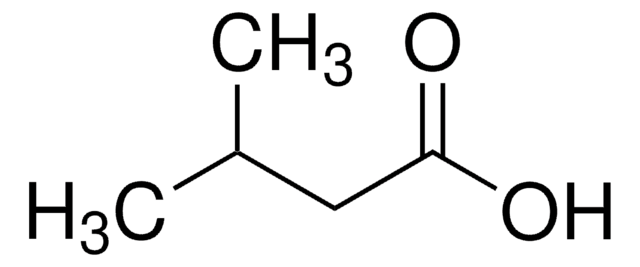
(S)-(+)-2-Hydroxy-3-methylbutyric acid
99%, optical purity ee: 99% (GLC)
Synonym(s):
L-α-Hydroxyisovaleric acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)2CHCH(OH)CO2H
CAS Number:
Molecular Weight:
118.13
Beilstein:
1721140
MDL number:
UNSPSC Code:
51113400
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
optical activity
[α]20/D +19°, c = 1 in chloroform
optical purity
ee: 99% (GLC)
bp
124-125 °C/13 mmHg (lit.)
mp
68-70 °C (lit.)
functional group
carboxylic acid
hydroxyl
SMILES string
CC(C)[C@H](O)C(O)=O
InChI
1S/C5H10O3/c1-3(2)4(6)5(7)8/h3-4,6H,1-2H3,(H,7,8)/t4-/m0/s1
InChI key
NGEWQZIDQIYUNV-BYPYZUCNSA-N
Related Categories
Application
A useful chiral building block for peptide synthesis. Used in the preparation of the cytotoxic didemnin cyclopeptides and of the antineoplastic agent dolastatin 15.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Journal of the American Chemical Society, 112, 7659-7659 (1990)
O Arad et al.
Biopolymers, 29(12-13), 1633-1649 (1990-10-01)
Depsipeptide analogues of peptide sequences can help in elucidating the role of specific hydrogen bonds in determining the conformation in peptides. The repeating pentapeptide and hexapeptide sequences of elastin have been suggested to contain a type II beta-turn with a
Schmidt, U. et al.
Synthesis, 294-294 (1991)
Pettit, G.R. et al.
Journal of the American Chemical Society, 113, 6692-6692 (1991)
Hye-Ran Yoon
Archives of pharmacal research, 30(3), 387-395 (2007-04-12)
A rapid dried-filter paper plasma-spot analytical method was developed to quantify organic acids, amino acids, and glycines simultaneously in a two-step derivatization procedure with good sensitivity and specificity. The new method involves a two-step trimethylsilyl (TMS) - trifluoroacyl (TFA) derivatization
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service