All Photos(2)
About This Item
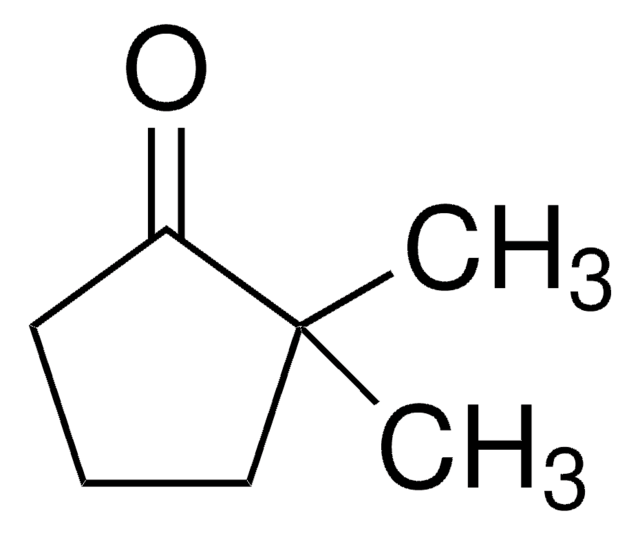
Linear Formula:
(CH3)2C6H8(=O)
CAS Number:
Molecular Weight:
126.20
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
92%
refractive index
n20/D 1.448 (lit.)
bp
169-170 °C/768 mmHg (lit.)
mp
−20 °C (lit.)
density
0.912 g/mL at 25 °C (lit.)
SMILES string
CC1(C)CCCCC1=O
InChI
1S/C8H14O/c1-8(2)6-4-3-5-7(8)9/h3-6H2,1-2H3
InChI key
KNSPBSQWRKKAPI-UHFFFAOYSA-N
Related Categories
General description
2,2-Dimethylcyclohexanone is a sterically hindered ketone. Mg-TiCl4-catalyzed CH2-transfer reaction of 2,2-dimethylcyclohexanone with CH2Cl2 is reported.
Application
2,2-Dimethylcyclohexanone may be used in the preparation of 6,6-dimethyl-1-vinylcyclohexene.
Other Notes
Remainder 2-methylcyclohexanone
Signal Word
Warning
Hazard Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
122.0 °F - closed cup
Flash Point(C)
50 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Tu-Hsin Yan et al.
Organic letters, 6(26), 4961-4963 (2004-12-21)
[reaction: see text] This Mg-TiCl4-promoted CH2-transfer reaction of CH2Cl2 represents an extremely simple, practical, and efficient methylenation of a variety of ketones and aldehydes, especially in enolizable or sterically hindered ketones such as 2,2-dimethylcyclohexanone, camphor, and fenchone.
Synthesis and antioxidant activity of rosmariquinone and several analogues.
Hall CA, et al.
Journal of Agricultural and Food Chemistry, 46(4), 1303-1310 (1998)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![[Ir{dFCF3ppy}2(bpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/180/924/79119ac4-7d62-429d-b23d-a14c012c6050/640/79119ac4-7d62-429d-b23d-a14c012c6050.png)