All Photos(1)
About This Item
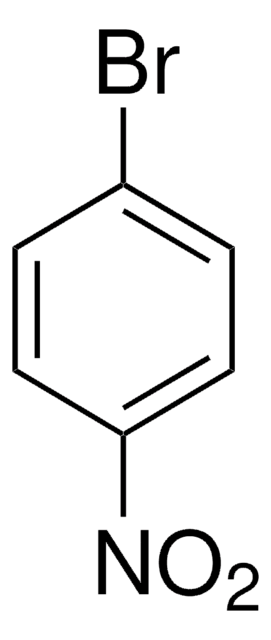
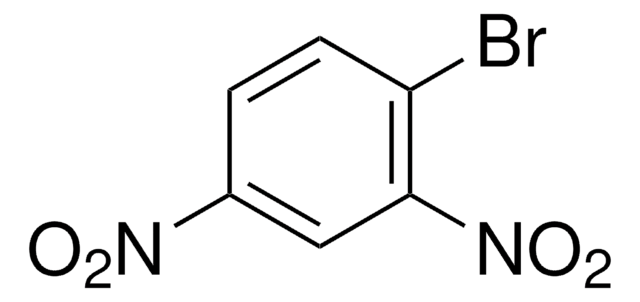
Linear Formula:
BrC6H4NO2
CAS Number:
Molecular Weight:
202.01
Beilstein:
2044109
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
bp
261 °C (lit.)
mp
40-42 °C (lit.)
storage temp.
2-8°C
SMILES string
[O-][N+](=O)c1ccccc1Br
InChI
1S/C6H4BrNO2/c7-5-3-1-2-4-6(5)8(9)10/h1-4H
InChI key
ORPVVAKYSXQCJI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
1-Bromo-2-nitrobenzene undergoes palladium[0]-mediated Ullmann cross-coupling reaction with a range of β-halo-enals, -enones or -esters to afford the corresponding β-aryl derivatives. Palladium[0]-mediated Ullmann cross-coupling reaction of 1-bromo-2-nitrobenzene with β-bromo-α,β-unsaturated aldehydes is reported.
Application
1-Bromo-2-nitrobenzene may be used in the preparation of:
- 4-methoxy-2′-nitrodiphenyl ether
- 1-methoxy-3,5-bis-(2-nitro-phenoxy)benzene
- 5-hydroxy-3-methoxy-2′-nitrodiphenyl ether
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
233.6 °F - closed cup
Flash Point(C)
112 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Martin G Banwell et al.
Organic letters, 6(16), 2741-2744 (2004-07-30)
Palladium[0]-mediated Ullmann cross-coupling of 1-bromo-2-nitrobenzene (1 R = H) and its derivatives with a range of beta-halo-enals, -enones, or -esters readily affords the corresponding beta-aryl derivatives, which are converted into the corresponding quinolines, 2-quinolones, phenanthridines, or 6(5H)-phenanthridinones on reaction with
New protocols for the synthesis of 3, 4-annulated and 4-substituted quinolines from ?-bromo-a, ?-unsaturated aldehydes and 1-bromo-2-nitrobenzene or 2-bromoacetanilide.
Some S, et al.
Tetrahedron Letters, 48(20), 3609-3612 (2007)
4-Hydroxy-2'-Nitrodiphenyl Ether Analogues as Novel Tyrosinase Inhibitors.
Sapkota K, et al.
Bull. Korean Chem. Soc., 31(5), 1319-1319 (2010)
Luka A Wright et al.
Dalton transactions (Cambridge, England : 2003), 44(16), 7230-7241 (2015-03-20)
The 2-(2′-aniline)-6-imine-pyridines, 2-(C6H4-2′-NH2)-6-(CMe=NAr)C5H3N (Ar = 4-i-PrC6H4 (HL1a), 2,6-i-Pr2C6H3 (HL1b)), have been synthesised via sequential Stille cross-coupling, deprotection and condensation steps from 6-tributylstannyl-2-(2-methyl-1,3-dioxolan-2-yl)pyridine and 2-bromonitrobenzene. The palladium(II) acetate N,N,N-pincer complexes, [{2-(C6H4-2′-NH)-6-(CMe=NAr)C5H3N}Pd(OAc)] (Ar = 4-i-PrC6H4 (1a), 2,6-i-Pr2C6H3 (1b)), can be prepared by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service