362069
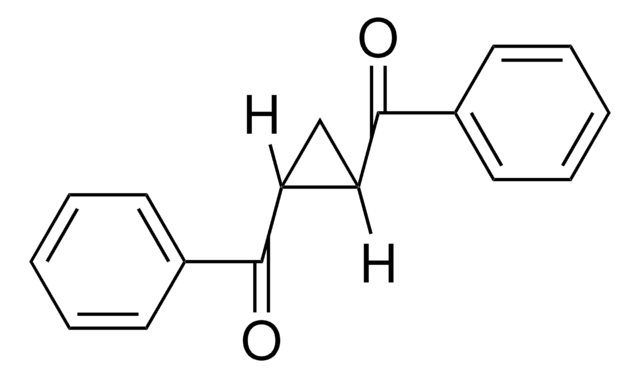
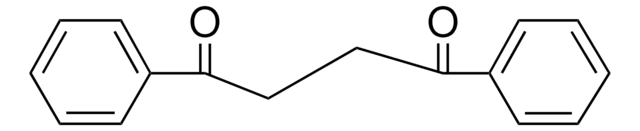
1,3-Dibenzoylpropane
98%
Synonym(s):
1,5-Diphenyl-1,5-pentanedione
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CO(CH2)3COC6H5
CAS Number:
Molecular Weight:
252.31
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
66-68 °C (lit.)
functional group
ketone
phenyl
SMILES string
O=C(CCCC(=O)c1ccccc1)c2ccccc2
InChI
1S/C17H16O2/c18-16(14-8-3-1-4-9-14)12-7-13-17(19)15-10-5-2-6-11-15/h1-6,8-11H,7,12-13H2
InChI key
YOLLTWVIOASMFW-UHFFFAOYSA-N
Related Categories
General description
1,3-Dibenzoylpropane is a 1,3-diaroylpropane. 1,3-Dibenzoylpropane is formed during hydroxocobalt(III) Schiff base complexes catalyzed selective aldol reaction of dibenzoylmethanes with formaldehyde in methanol.
Application
1,3-Dibenzoylpropane may be used in the electrochemical synthesis of cis-1,2-diphenyl-1,2-cyclopentanediol, via reduction in acetonitrile.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Highly selective aldol reaction of dibenzoylmethanes with formaldehyde catalyzed by cobalt Schiff base complex under neutral conditions.
Maruyama K, et al.
Tetrahedron Letters, 36(31), 5609-5612 (1995)
Electrochemical cyclization: II. Intramolecular pinacolization of 1, 3-dibenzoylpropane in acetonitrile.
Ammar F, et al.
J. Electroanal. Chem. Interfac. Electrochem., 53(3), 407-416 (1974)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![2,3,6,7-Tetrahydro-8-hydroxy-1H,5H-benzo[ij]quinolizine-9-carboxaldehyde 98%](/deepweb/assets/sigmaaldrich/product/structures/166/830/a0d9a84a-5623-41a1-a54b-3b0272e5b28c/640/a0d9a84a-5623-41a1-a54b-3b0272e5b28c.png)