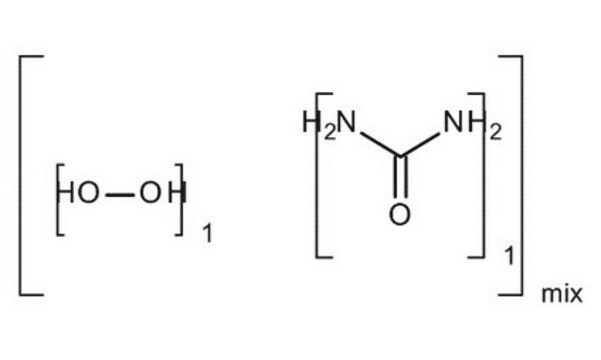
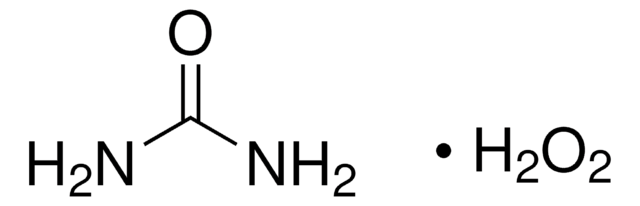
289132
Urea hydrogen peroxide
97%
Synonym(s):
Hydrogen peroxide–Urea adduct, Carbamide Per hydrate, Carbamide peroxide, Percarbamide, Urea hydrogen peroxide
About This Item
Recommended Products
vapor pressure
23.3 mmHg ( 30 °C)
Quality Level
Assay
97%
form
solid
reaction suitability
reagent type: oxidant
mp
90-93 °C (lit.)
functional group
amine
storage temp.
2-8°C
SMILES string
OO.NC(N)=O
InChI
1S/CH4N2O.H2O2/c2-1(3)4;1-2/h(H4,2,3,4);1-2H
InChI key
AQLJVWUFPCUVLO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- In the conversion of sulfides to sulfoxides or sulfones under solvent free condition.
- In the conversion of nitrogen heterocycles to their corresponding N-oxides.
- In the epoxidation of various alkene in combination with number of carboxylic anhydrides and disodium hydrogen phosphate in anhydrous organic solvents.
- In the Baeyer–Villiger oxidation reaction where in aldehydes and ketones undergo oxidation to produce esters and lactones using various peroxycarboxylic acids.
Features and Benefits
- Inexpensive.
- Stable.
- Easy handling reagent.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Ox. Sol. 3 - Skin Irrit. 2
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service