All Photos(1)
About This Item
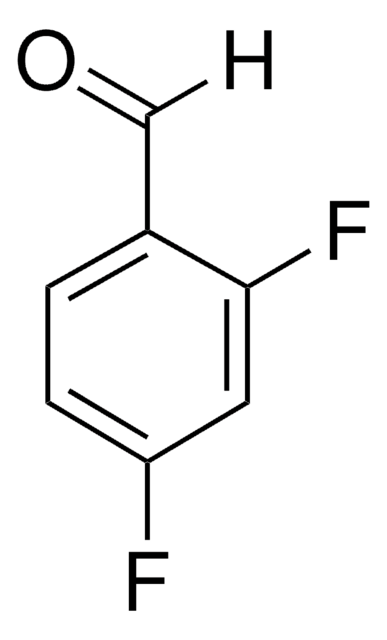
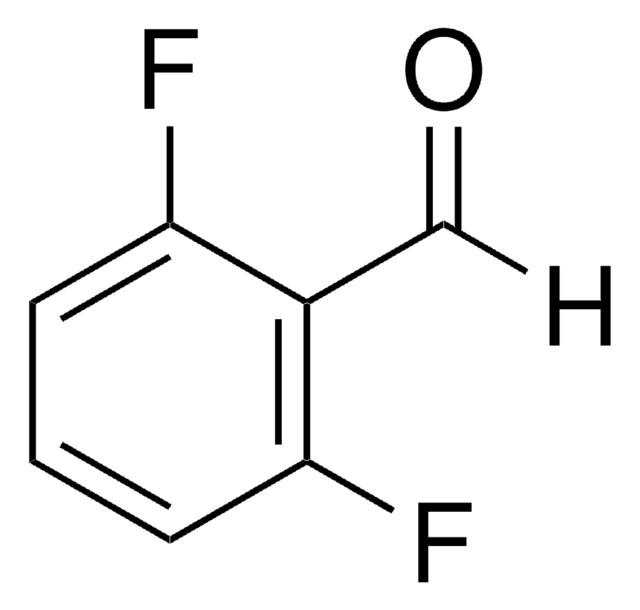
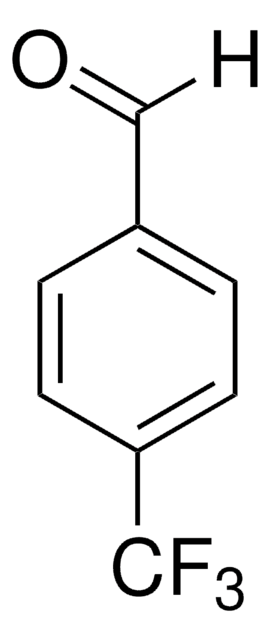
Linear Formula:
F2C6H3CHO
CAS Number:
Molecular Weight:
142.10
Beilstein:
2241231
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.5 (lit.)
density
1.288 g/mL at 25 °C (lit.)
functional group
aldehyde
fluoro
SMILES string
[H]C(=O)c1ccc(F)c(F)c1
InChI
1S/C7H4F2O/c8-6-2-1-5(4-10)3-7(6)9/h1-4H
InChI key
JPHKMYXKNKLNDF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3,4-Difluorobenzaldehyde was used in the synthesis of 3-benzylidene 20,29-dihydrobetulinic acid derivatives.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
156.2 °F - DIN 51758
Flash Point(C)
69 °C - DIN 51758
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rama Mukherjee et al.
Bioorganic & medicinal chemistry letters, 14(12), 3169-3172 (2004-05-20)
New 3-O-acyl, 3-benzylidene, 3-hydrazone, 3-hydrazine, 17-carboxyacryloyl ester derivatives of betulinic acid (2-6, 8-11, 13, 17, 18, 21, and 22) were synthesized and evaluated in vitro for anti-angiogenic activity on endothelial cell cytotoxicity, specificity, and tube-formation ability. All derivatives reported here
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 265160-25G | 4061838348258 |
| 265160-5G | 4061826124901 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service