252522
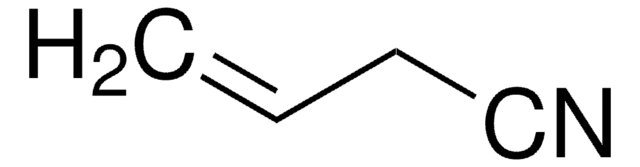
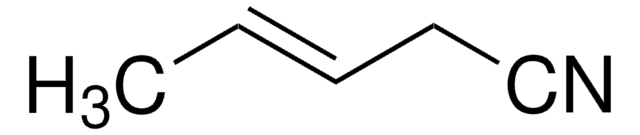
Crotononitrile, mixture of cis and trans
99%
Synonym(s):
2-Butenenitrile, mixture of cis and trans
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
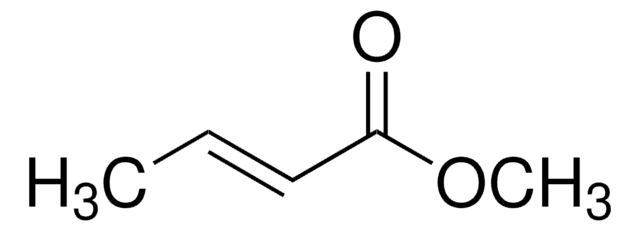
CH3CH=CHCN
CAS Number:
Molecular Weight:
67.09
Beilstein:
1719747
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.419 (lit.)
bp
120-121 °C (lit.)
density
0.824 g/mL at 25 °C (lit.)
functional group
nitrile
SMILES string
C\C=C\C#N
InChI
1S/C4H5N/c1-2-3-4-5/h2-3H,1H3/b3-2+
InChI key
NKKMVIVFRUYPLQ-NSCUHMNNSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Neurotoxic properties of crotononitrile have been investigated. Mechanism of the Baylis-Hillman reaction of crotononitrile and benzaldehyde in the presence of 1,4-diazabicyclo[2,2,2]octane (catalyst) has been investigated.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
60.8 °F - closed cup
Flash Point(C)
16 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Effect of solvent, pressure and catalyst on the E/Z-selectivity in the Baylis-Hillman reaction between crotononitrile and benzaldehyde.
van Rozendaal ELM, et al.
Tetrahedron, 49(31), 6931-6936 (1993)
Pere Boadas-Vaello et al.
Toxicology and applied pharmacology, 225(3), 310-317 (2007-09-20)
Several alkylnitriles are toxic to sensory systems, including the vestibular system, through yet undefined mechanisms. This study addressed the hypothesis that the vestibular toxicity of cis-crotononitrile depends on CYP2E1-mediated bioactivation. Wild-type (129S1) and CYP2E1-null female mice were exposed to cis-crotononitrile
Eduardo Balbuena et al.
Toxicology and applied pharmacology, 187(2), 89-100 (2003-03-22)
The neurotoxic compound crotononitrile has two isomeric forms, cis and trans. We compared the effects of these two isomers isolated by distillation from the commercially available mixture. Adult male Long-Evans rats were administered vehicle control, cis-crotononitrile (80, 100, and 120
Nathan A Owston et al.
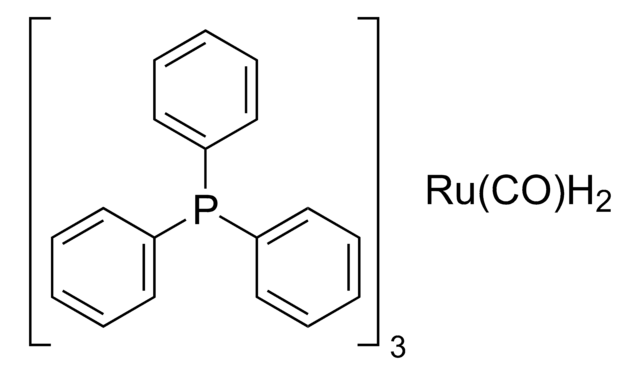
Chemical communications (Cambridge, England), (5)(5), 624-625 (2008-01-23)
The oxidation of alcohols in the presence of methanol has been achieved using a ruthenium catalyst with crotononitrile as the hydrogen acceptor.
Rafat M Mohareb et al.
Acta pharmaceutica (Zagreb, Croatia), 58(4), 429-444 (2008-12-24)
Condensation of beta-amino-alpha,gamma-dicyanocrotononitrile (1) with acetophenone gave 2-amino-4-phenylpenta-1,3-diene-1,1,3-tricarbonitrile (2). The latter product was used in a series of heterocyclization reactions with different reagents such as diazonium salts, hydrazines, hydroxylamines and elemental sulfur to give pyridazine, pyrazole, isoxazole and thiophene derivatives
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service