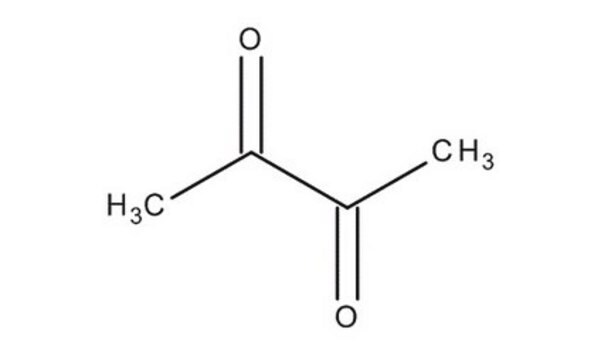
241962
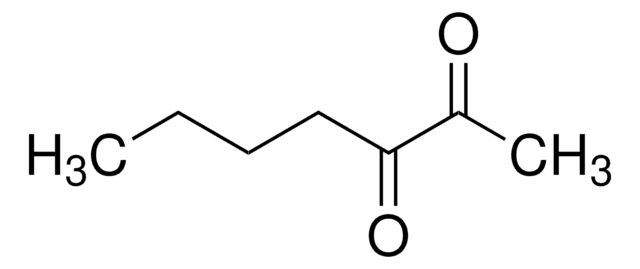
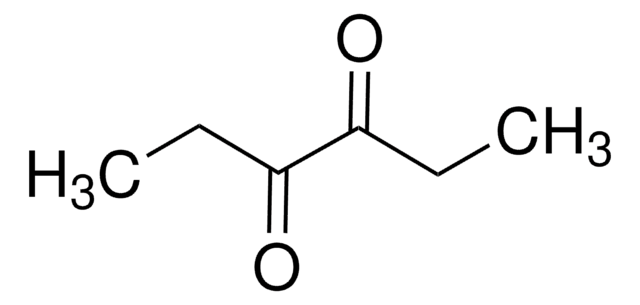
2,3-Pentanedione
97%
Synonym(s):
Acetylpropionyl
About This Item
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.404 (lit.)
bp
110-112 °C (lit.)
mp
−52 °C (lit.)
density
0.957 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CCC(=O)C(C)=O
InChI
1S/C5H8O2/c1-3-5(7)4(2)6/h3H2,1-2H3
InChI key
TZMFJUDUGYTVRY-UHFFFAOYSA-N
Gene Information
human ... ACHE(43) , BCHE(590) , CES1(1066)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Bisphenol derivatives by acid-catalyzed condensation reaction with phenols.
- 2-Ethyl-3-methyl-1H-indole by Pd-catalyzed reaction with aniline under reductive conditions.
- 2-Ethyl-3-methylquinoxaline by condensation reaction with o-phenylenediamine using citric acid as a catalyst.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2 - Skin Sens. 1B - STOT RE 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
66.2 °F - open cup
Flash Point(C)
19 °C - open cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
-Cymene; 2,5-Dimethylpyrrole; Acetoin, ≥96%, FCC, FG; 2,5-Dimethylpyrazine; 2,6-Dimethylpyrazine; 2-Ethylpyrazine, ≥98%, FG; 2,3-Dimethylpyrazine; 4-Heptanone; 3-Ethylpyridine; 2,3,5-Trimethylpyrazine; Furfural; Pyrrole; Furfuryl acetate; Linalool; Linalyl acetate; 5-Methylfurfural; γ-Butyrolactone; 2-Acetyl-1-methylpyrrole; Furfuryl alcohol; 2-Acetylpyrrole; Pyrrole-2-carboxaldehyde
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service