All Photos(1)
About This Item
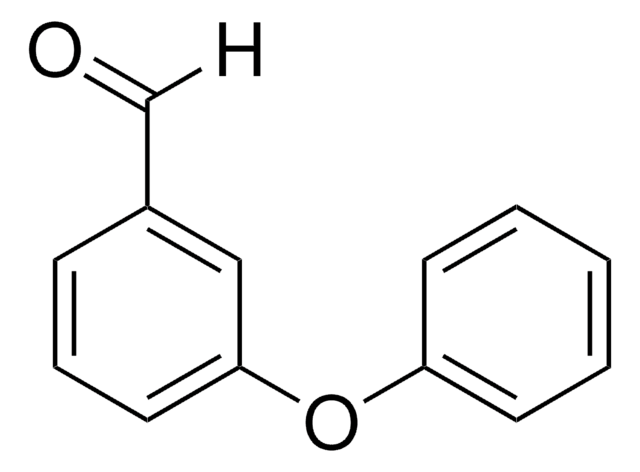
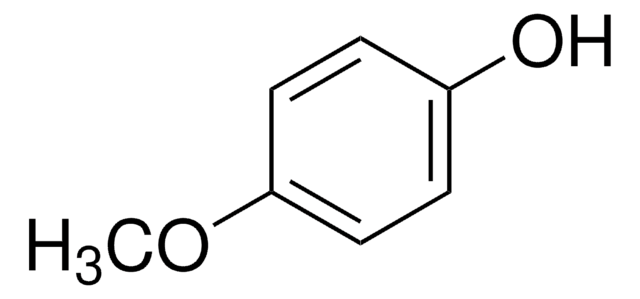
Linear Formula:
C6H5OC6H4CHO
CAS Number:
Molecular Weight:
198.22
Beilstein:
1947841
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.611 (lit.)
bp
185 °C/14 mmHg (lit.)
mp
24-25 °C (lit.)
density
1.132 g/mL at 25 °C (lit.)
functional group
aldehyde
phenoxy
SMILES string
O=Cc1ccc(Oc2ccccc2)cc1
InChI
1S/C13H10O2/c14-10-11-6-8-13(9-7-11)15-12-4-2-1-3-5-12/h1-10H
InChI key
QWLHJVDRPZNVBS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-Phenoxybenzaldehyde was used in the synthesis of:
- spirodiketopiperazine derivatives
- benzoxazoles
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kelly J McClure et al.
Bioorganic & medicinal chemistry letters, 16(7), 1924-1928 (2006-01-31)
In a recent paper, [Arienti, K. L.; Brunmark, A.; Axe, F. U.; McClure, K. M.; Lee, A.; Blevitt, J.; Neff, D. K.; Huang, L.; Crawford, S.; Chennagiri, R. P.; Karlsson, L.; Brietenbucher, J. G. J. Med. Chem.2005, 48, 1873], we
Spirodiketopiperazine-based CCR5 antagonists: Lead optimization from biologically active metabolite.
Rena Nishizawa et al.
Bioorganic & medicinal chemistry letters, 17(3), 727-731 (2006-11-23)
Hydroxylated derivatives were designed and synthesized based on the information of oxidative metabolites. Compounds derived from beta-substituted (2R,3R)-2-amino-3-hydroxypropionic acid showed improved inhibitory activities against the binding of MIP-1alpha to human CCR5, compared with the non-hydroxylated derivatives and the other isomers.
Vikas N Telvekar et al.
Bioorganic & medicinal chemistry letters, 22(1), 649-652 (2011-11-15)
A series of structurally novel, substituted 2-(2-(4-aryloxybenzylidene) hydrazinyl)benzothiazole derivatives incorporating 2-hydrazinyl benzothiazole and 4-(aryloxy)benzaldehyde were designed and synthesized using molecular hybridization approach. All the synthesized compounds exhibited promising activity (MIC 1.5-29.00μg/ml) against Mycobacteriumtuberculosis H37Rv strains of using REMA. Five of
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 211265-1G | |
| 211265-25G | 4061836683061 |
| 211265-5G | 4061836683078 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service