All Photos(1)
About This Item
Empirical Formula (Hill Notation):
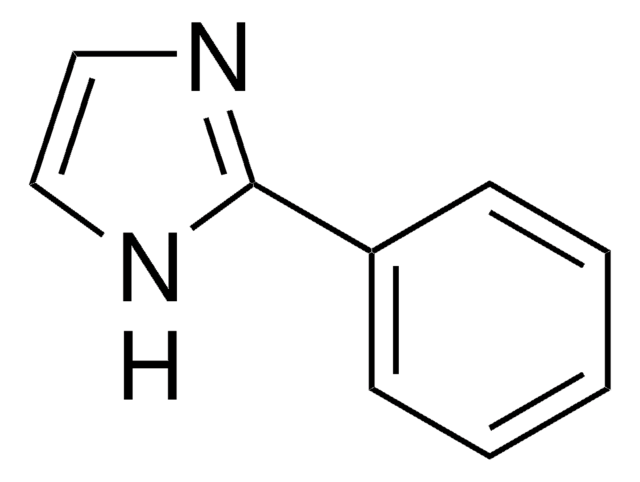
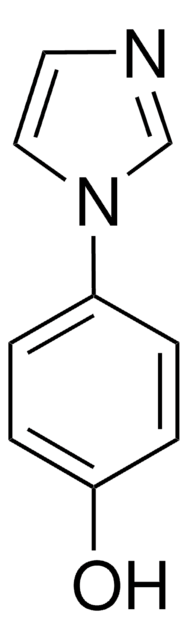
C9H8N2
CAS Number:
Molecular Weight:
144.17
Beilstein:
2969
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
128-131 °C (lit.)
solubility
acetone: soluble 25 mg/mL, clear, colorless to yellow (typical)
functional group
phenyl
SMILES string
c1ccc(cc1)-c2c[nH]cn2
InChI
1S/C9H8N2/c1-2-4-8(5-3-1)9-6-10-7-11-9/h1-7H,(H,10,11)
InChI key
XHLKOHSAWQPOFO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
4-Phenylimidazole was used to investigate the protein-ligand interactions in cytochrome P450 from the thermoacidophile Picrophilus torridus. It was used as heme ligand during the crystallization of recombinant human indoleamine 2,3-dioxygenase. It was used in the synthesis of complexes of copper and cobalt.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M Spatzenegger et al.
Molecular pharmacology, 59(3), 475-484 (2001-02-17)
The molecular basis for reversible inhibition of rabbit CYP2B4 and CYP2B5 and rat CYP2B1 by phenylimidazoles was assessed with active-site mutants and new three-dimensional models based on the crystal structure of CYP2C5. 4-Phenylimidazole was 17- to 32-fold more potent toward
Danni L Harris et al.
Proteins, 55(4), 895-914 (2004-05-18)
The molecular origins of temperature-dependent ligand-binding affinities and ligand-induced heme spin state conversion have been investigated using free energy analysis and DFT calculations for substrates and inhibitors of cytochrome P450 2B4 (CYP2B4), employing models of CYP2B4 based on CYP2C5(3LVdH)/CYP2C9 crystal
Y K Li et al.
Biochimica et biophysica acta, 999(3), 227-232 (1989-12-21)
Over 25 nitrogen-containing heterocycles were tested as inhibitors of sweet almond beta-glucosidase (EC 3.2.1.21). Among the most potent of these are some imidazole derivatives. The pH dependence indicates that the unprotonated inhibitor binds most tightly to the catalytically active species
Spectral and metabolic properties of liver microsomes from imidazole-pretreated rabbits.
K K Hajek et al.
Biochemical and biophysical research communications, 108(2), 664-672 (1982-09-30)
Y K Li et al.
Journal of biochemistry, 123(3), 416-422 (1998-05-30)
Series of 4-arylimidazoles, omega-N-acylhistamines and 4-(omega-phenylalkyl)imidazoles were synthesized in order to probe the active site topology of sweet almond beta-glucosidase. These imidazole derivatives were shown to be very powerful competitive inhibitors. Among the 20 tested compounds, omega-N-benzoylhistamine and 4-(3'-phenylpropyl)imidazole are
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service