136123
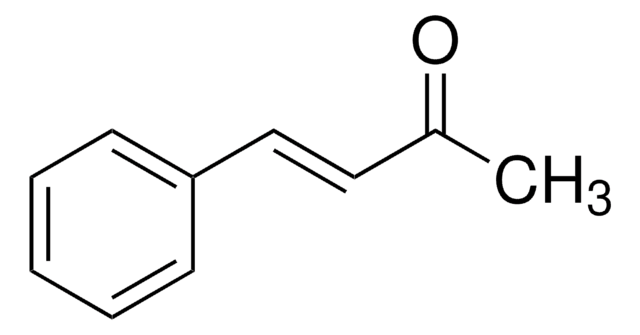
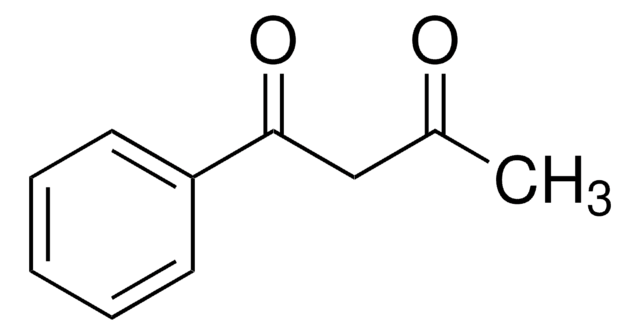
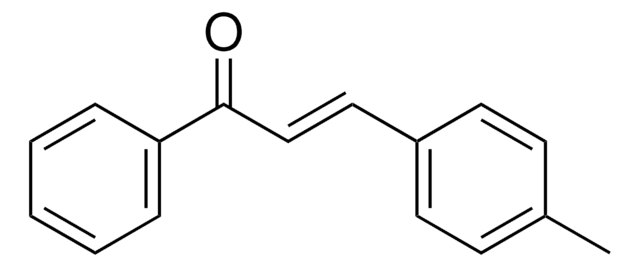
trans-Chalcone
97%
Synonym(s):
Benzylideneacetophenone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH=CHCOC6H5
CAS Number:
Molecular Weight:
208.26
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
bp
208 °C/25 mmHg (lit.)
mp
55-57 °C (lit.)
functional group
ketone
phenyl
SMILES string
[H]\C(=C(\[H])C(=O)c1ccccc1)c2ccccc2
InChI
1S/C15H12O/c16-15(14-9-5-2-6-10-14)12-11-13-7-3-1-4-8-13/h1-12H/b12-11+
InChI key
DQFBYFPFKXHELB-VAWYXSNFSA-N
Gene Information
human ... IL1B(3553)
rat ... Ar(24208)
Related Categories
General description
trans-Chalcone is an open chain flavonoid that may prevent lung and forestomach cancer.
Application
trans-Chalcone was used in the synthesis of cis and trans diphenyl cyclopropane. It was also used in screening of surface adsorbed species of isobutybenzene and 4-isobutylacetophenone on bulk fosfotungstic Wells-Dawson acid (H6P2W18O62.xH2O).
Biochem/physiol Actions
trans-Chalcone exhibits antifungal activity against Trichophyton rubrum. It is inhibitor of fatty acid synthase and α-amylase. It induces programmed cell death due to reduced mitochondrial transmembrane potential in Arabidopsis thaliana roots.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sylvie Ducki et al.
Bioorganic & medicinal chemistry, 17(22), 7698-7710 (2009-10-20)
The alpha-methyl chalcone SD400 is a potent inhibitor of tubulin assembly and possesses potent anticancer activity. Various chalcone analogues were synthesized and evaluated for their cell growth inhibitory properties against the K562 human chronic myelogenous leukemia cell line (SD400, IC(50)
Wattenberg L.W., et al.
Cancer Letters, 15, 165-165 (1994)
Daniela Batovska et al.
European journal of medicinal chemistry, 44(5), 2211-2218 (2008-07-01)
A large series of chalcones were synthesized and studied against Staphylococcus aureus and Escherichia coli. Chalcones were either unsubstituted in ring A or possessed 4'-chloro or 3',4',5'-trimethoxy groups. Their other ring B was variously substituted. It was found that the
Saeed Attar et al.
Bioorganic & medicinal chemistry, 19(6), 2055-2073 (2011-02-26)
A series of 30 organic chlacones and 33 ferrocenyl (Fc) chalcones were synthesized and characterized by melting point, elemental analysis, spectroscopy ((1)H NMR and FTIR) and, in two cases, by X-ray crystallography. The biological activity of each compound (10(-4)M in
Asha Budakoti et al.
European journal of medicinal chemistry, 44(3), 1317-1325 (2008-04-02)
In an effort to develop potent antiamoebic agents, we have synthesized chalcones (1-8), amino-5-substituted-(3-phenyl(2-pyrazolinyl))methane-1-thione derivatives (1a-8a) and 2-(5-substituted-3-phenyl-2-pyrazolinyl)-1,3-thiazolino[5,4-b]quinoxaline derivatives (1b-8b) and evaluated for their in vitro antiamoebic activity against HM1:IMSS strain of E. histolytica. All the compounds were characterized by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service