Fast and Accurate LC-MS Analysis of Vitamin D Metabolites Using Ascentis® Express F5 HPLC Columns
Craig R. Aurand, David S. Bell
Reporter US Volume 29.2
Introduction
Vitamin D deficiency has become a topic of interest in recent publications.1-3 Vitamin D, along with calcium, promotes proper bone growth in children and aids in the prevention of osteoporosis in older adults. Vitamin D is present in two forms, Vitamin D3 and Vitamin D2. D3 is produced after ultraviolet light-stimulated conversion of 7-dehydrocholesterol in the skin.3 Vitamin D2 is derived from plant sources. Both D2 and D3 are metabolized in the liver to form 25-hydroxyvitamin D2 (25-OH D2) and 25-hydroxyvitamin D3 (25-OH D3), respectively. In addition, biologically inactive 3-epi analogs of 25-OH D2 and 25-OH D3 have been reported, especially in young children.3 The levels of the 25-hydroxy metabolites are routinely measured for diagnostic assessment of vitamin D related diseases; however, recent studies have indicated that separation from the inactive 3-epi analogs may provide more accurate information for treatment and prevention. Analytical methods that can accurately quantitate both of the 25-hydroxyvitamin D analytes in the presence of 3-epi analogs may become essential for diagnosis and monitoring of patients with vitamin D disorders.

Figure 1. Vitamin D 25-hydroxy Metabolite Structures

Vitamin D3 is produced in the skin after exposure to sunlight
HPLC analysis of 25-OH D2 and 25-OH D3 is classically performed using C18 stationary phases. Under such conditions, the 3-epi analogs are not resolved and thus are included in the overall reported value. Recently, Phinney, et al., reported the use of a cyano column for the effective separation of the 25-OH and the 3-epi forms for use in reference measurement procedures.1 Although effective, the conditions necessitate a run time of better than 40 minutes limiting its utility for routine high-throughput analyses.
As an outcome of some recent application development efforts, it was observed that a pentafluorophenyl (PFP, Ascentis Express F5) stationary phase provided increased selectivity toward 25-OH D3 and the corresponding 3-epi analog relative to reported methods. This report provides a brief synopsis of continuing efforts to assess the potential impact of this additional selectivity on routine clinical vitamin D diagnostics.
Discussion
The structures of the vitamin D analytes are shown in Figure 1, while the initial separation of 25-OH D3 and 3-epi-25-OH D3 using the fluorinated phase is presented in Figure 2. The separation demonstrates that selectivity between the analogs can be achieved in under 10 minutes, whereas separation using a cyano column required nearly 40 minutes.
The ultimate goal for this separation is likely to entail the use of mass spectrometry to reach the desired levels of quantification and specificity. With this in mind the initial conditions were adopted for fast LC-MS methodology. Figure 3 shows some preliminary results indicating that 25-OH D3 and 3-epi-25-OH D3 can be rapidly resolved. 25-OH D2 and 3-epi-25-OH D3 coelute under these high throughput conditions, however they are easily resolved by mass response. The methodology thus enables quantification of all three components in one analysis.
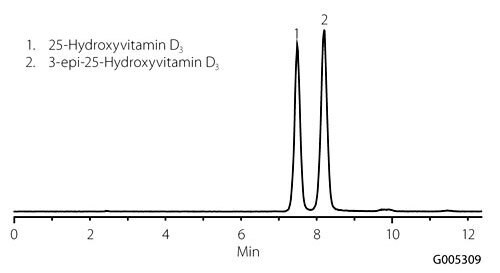
Figure 2. Separation of 25-Hydroxyvitamin D3 and 3-epi 25-Hydroxyvitamin D3 Using Ascentis Express F5

Figure 3. Fast, LC-MS Analysis of Vitamin D Metabolites Using Ascentis Express F5
Conclusions
Separation of the biologically inactive 3-epi analog may serve to provide improved data in support of vitamin D related clinical diagnostics and treatment. The pentafluorophenyl stationary phase has been shown to provide superior selectivity for the separation of the closely related 25-OH D3 and 3-epi-25-OH D3 as compared to methods reported in the literature. Initial efforts to show selectivity in a fast, LC-MS system provides promising evidence for implementation in real-world situations. As low analyte mass response and interferences from sample matrices may pose additional analysis problems, work is currently underway to further explore both chromatographic and sample preparation procedures in an attempt to optimize both speed and sensitivity.
References
To continue reading please sign in or create an account.
Don't Have An Account?