Fast LC-MS-MS Analysis of 25-Hydroxyvitamin D2 and 25-Hydroxyvitamin D3
Contributed Article
The following was generated with the assistance of an outside source using our products. Technical content was generated and provided by:
Jesse C. Seegmiller and Keith J. Goodman
AB SCIEX Framingham, MA
Abstract
A robust method for analyzing 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in serum using a QTRAP® 5500 and an MPX™-2 High Throughput multiplexed LC-MS-MS system is presented. Sample preparation was simplified in order to accommodate automated liquid handling systems and to minimize the time commitment needed by clinical staff. With multiplexing, sample results were achieved in less than 1.5 minutes per sample. Accuracy and linearity was demonstrated for 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 over a concentration range 0.5-100 ng/mL in serum. This dynamic range was achieved without the use of solid phase extraction, online extraction, or any other type of sample concentration steps.
Introduction
Heightened interest in vitamin D is related to an observed trend of increased vitamin D insufficiency and its role in human health.1 Vitamin D is derived in vivo primarily from UVB radiation impacting the skin where 7-dehydrocholesterol is converted to cholecalciferol (vitamin D3) through a photolytic, nonenzymatic reaction. Smaller amounts of vitamin D are acquired through diet, including vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol) from animal and plant sources, respectively. Vitamin D3 is metabolized to 25-hydroxyvitamin D3 in the liver and then to the active form 1,25-dihydroxyvitamin D3 in the kidney. Circulating levels of 1,25-dihydroxyvitamin D are tightly controlled and serve to modulate expression of specific genes through the vitamin D receptor.
Vitamin D acts to promote intestinal calcium and phosphate absorption and also controls their liberation from bone. In addition to rickets and osteomalacia, low serum 25-hydroxyvitamin D3 and D2, has been linked to hypertension, autoimmune diseases, and cancer.2,3
The most abundant metabolite of vitamin D is 25-hydroxyvitamin D and it is considered one of the best indicators of vitamin D status. Accurately measuring 25-hydroxyvitamin D is necessary to adequately assess an individual’s vitamin D levels and to help determine the role of vitamin D in various diseases. Immunoassays suffer from high reagent cost, narrow dynamic range, and poor selectivity (inability to distinguish 25-hydroxyvitamin D3 from 25-hydroxyvitamin D2).4 These inadequacies of immunoassays make them unattractive for vitamin D analysis in patient samples.
Liquid chromatography tandem mass spectrometry (LC-MS-MS) is a highly sensitive and specific technique for the analysis of a wide range of compounds contained in biological matrices. The current generation of this technology is capable of reliably determining 25-hydroxyvitamin D concentrations across the entire clinical range. Coupled with liquid chromatography, accurate and precise measurements of both 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 can be routinely achieved using LC-MS-MS. The primary goal of this method was to use a simple sample preparation step while accommodating high throughput 25-hydroxyvitamin D runs, ~500/day, on a single LC-MS-MS system.
Sample Preparation
Protein precipitation consisted of combining 50 μL of serum with 100 μL acetonitrile in a 1.5 mL centrifuge tube. The sample was then centrifuged at 5,000 g for 5 minutes before transferring the supernatant into a sample vial for analysis.
MS/MS Conditions
The multiplexed LC system, MPX™, was coupled to an AB SCIEX QTRAP® 5500 LC-MS-MS system with a Turbo V™ source and Atmospheric Pressure Chemical Ionization (APCI) probe in positive ion mode. A total of 5 Multiple Reaction Monitoring (MRM) transitions were monitored, including 2 per compound (quantifier and qualifier) and one for the internal standard, 25-hydroxyvitamin D3-d6. Chromatographic conditions are listed in Figures 1 and 2.
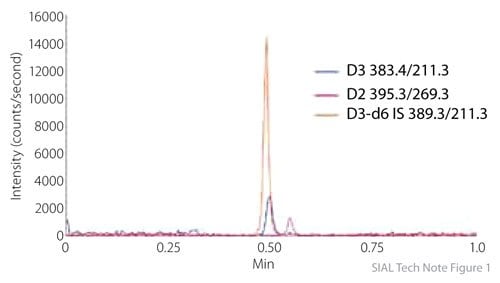
Figure 1.Initial Serum Extracted Sample of 350 Injections

Figure 2.Re-Injection of the First Sample after 350 Injections of Serum Extracted Samples Analyzed
Results and Discussion
A method suitable for quantification of 25-hydroxyvitamin D2 and D3 required only two minutes, prior to multiplexing. Figure 1 shows the separation of 25-hydroxyvitamin D2 and D3 and the internal standard. This is the first injection of an extracted serum sample on the Ascentis Express C18 column. Figure 2 shows the result of re-injecting this first sample after 350 serum extracted samples were run. Note that peak shape, retention, and signal response have not changed after the 350 injections. This indicates a very robust method which employed simple sample preparation (protein precipitation) prior to analysis. Quantitative results for serum samples are presented in Tables 1 and 2. These results show satisfactory precision and accuracy for a high-throughput method requiring minimal sample preparation.
*n= 8 injections/concentration (ng/mL), results were compiled from a 461 injection data set including matrix samples, standards, and controls.
*n= 8 injections/concentration (ng/mL), results were compiled from a 461 injection data set including matrix samples, standards, and controls.
Conclusion
A robust and reliable assay for 25-hydroxyvitamin D capable of handling high sample throughput observed in a clinical research laboratory was presented. Through the use of the AB SCIEX MPX™ system and the QTRAP® 5500 LC-MS-MS system in combination with Supelco’s Ascentis Express C18 column, laboratories can achieve 25-hydroxyvitamin D sample analysis times of <1.5 minutes per sample. This streamlined method can be implemented without the need for UHPLC systems or advanced knowledge of separation chemistry and achieves Limits of Quantitation (LOQ) of 1 ng/mL for vitamin D2 and D3.
References
To continue reading please sign in or create an account.
Don't Have An Account?