N1895
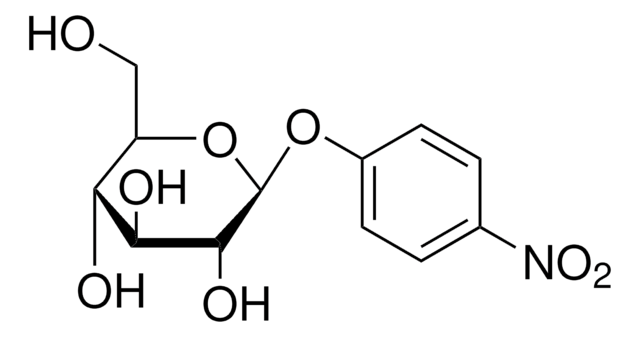
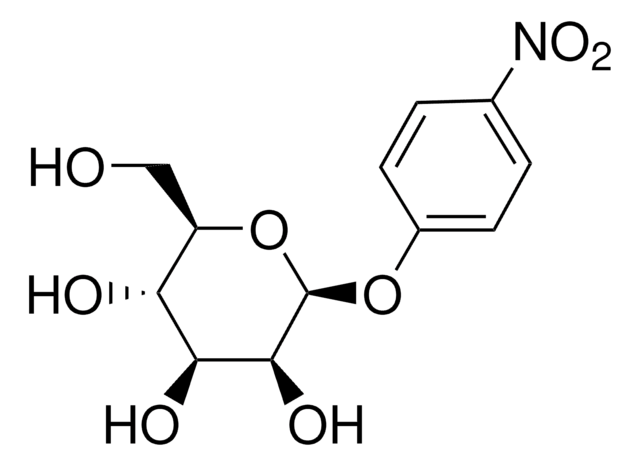
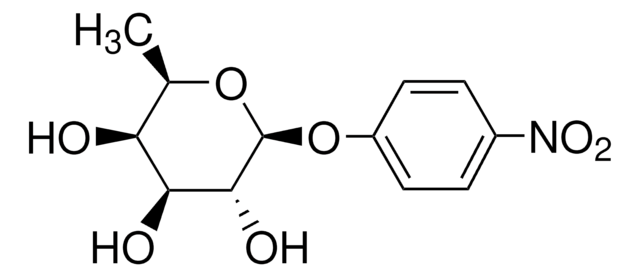
4-Nitrophenyl α-D-xylopyranoside
α-xylosidase substrate, ≥99% (HPLC), powder
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H13NO7
CAS Number:
Molecular Weight:
271.22
EC Number:
MDL number:
UNSPSC Code:
12352204
PubChem Substance ID:
NACRES:
NA.32
Recommended Products
Product Name
4-Nitrophenyl α-D-xylopyranoside, α-xylosidase substrate
Assay
≥99% (HPLC)
form
powder
solubility
methanol: soluble 20 mg/mL, clear, colorless to faintly yellow
storage temp.
−20°C
SMILES string
OC1COC(Oc2ccc(cc2)N(=O)=O)C(O)C1O
InChI
1S/C11H13NO7/c13-8-5-18-11(10(15)9(8)14)19-7-3-1-6(2-4-7)12(16)17/h1-4,8-11,13-15H,5H2
InChI key
MLJYKRYCCUGBBV-UHFFFAOYSA-N
Substrates
Chromogenic substrate for α-xylosidase
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Masaki Yanagishita et al.
Kokubyo Gakkai zasshi. The Journal of the Stomatological Society, Japan, 73(1), 20-25 (2006-04-25)
Biosynthesis of proteoglycans and glycosaminoglycans in the presence of p-nitrophenyl-xyloside was studied using a primary rat ovarian granulosa cell culture system. Addition of p-nitrophenyl-xyloside into cell culture medium caused about a 700% increase of [³⁵S]sulfate incorporation (ED50 at 0.03 mM)
T Bravman et al.
FEBS letters, 495(1-2), 115-119 (2001-04-27)
A beta-xylosidase from Bacillus stearothermophilus T-6 was cloned, overexpressed in Escherichia coli and purified to homogeneity. Based on sequence alignment, the enzyme belongs to family 39 glycoside hydrolases, which itself forms part of the wider GH-A clan. The conserved Glu160
Mária Mastihubová et al.
Carbohydrate research, 339(7), 1353-1360 (2004-04-29)
Di-O-acetates and mono-O-acetates of 4-nitrophenyl beta-D-xylopyranoside were prepared by use of lipase PS-30. Polarity of organic solvents and reaction time affected the regioselectivity of the di-O-acetylation as well as the yields of monoacetates. The kinetics of acetyl groups migration in
Peter Biely et al.
Biochimica et biophysica acta, 1770(4), 565-570 (2007-01-31)
Positional specificity of NodB-like domain of a multidomain xylanase U from Clostridium thermocellum (CtAxe) was investigated. Of three monoacetates of 4-nitrophenyl beta-d-xylopyranoside the acetylxylan esterase domain showed a clear preference for the 2-acetate. Moreover, the enzyme was significantly activated by
Siyuan Li et al.
Histochemistry and cell biology, 139(1), 59-74 (2012-08-23)
Chondroitin/dermatan sulphate (CS/DS) sulphation motifs on cell and extracellular matrix proteoglycans (PGs) within stem/progenitor cell niches are involved in modulating cell phenotype during the development of many musculoskeletal connective tissues. Here, we investigate the importance of CS/DS chains and their
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service