A9103
Anti-phospho-APP (pThr668) antibody produced in rabbit
affinity isolated antibody, aqueous glycerol solution, 10 blots
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
rabbit
Quality Level
conjugate
unconjugated
antibody form
affinity isolated antibody
antibody product type
primary antibodies
clone
polyclonal
form
aqueous glycerol solution
usage
10 blots
mol wt
antigen 100-140 kDa
species reactivity
human
technique(s)
western blot: 1:1000 using CAD cells transfected with wild type vs. T668A mutant APP.
UniProt accession no.
storage temp.
−20°C
Gene Information
human ... APP(351)
mouse ... App(11820)
rat ... App(54226)
General description
Amyloid precursor proteins (APPs) are transmembrane glycoproteins that are found in a wide range of tissues. APPs have 3 main isoforms, namely, APP695, APP751 and APP770 that are derived from alternative splicing events in cells. Accumulation of the cleavage products of APP, such as the β-amyloid peptide, can cause Alzheimer′s disease.
Rabbit Anti-phospho-APP (pThr668) antibody binds to human APP phosphorylated at Thr668. Mouse, rat and frog APP are 100% homologous.
Rabbit Anti-phospho-APP (pThr668) antibody binds to human APP phosphorylated at Thr668. Mouse, rat and frog APP are 100% homologous.
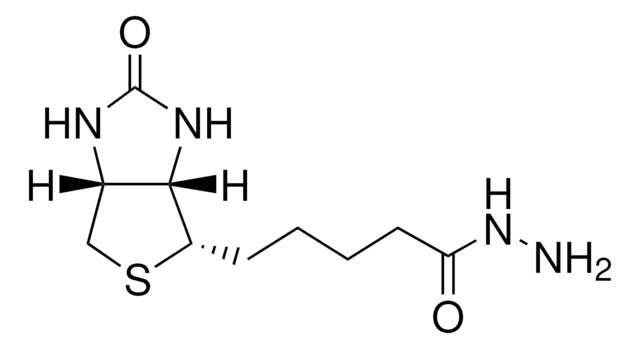
Immunogen
synthetic phosphopeptide corresponding to the region of APP containing threonine 688.
Application
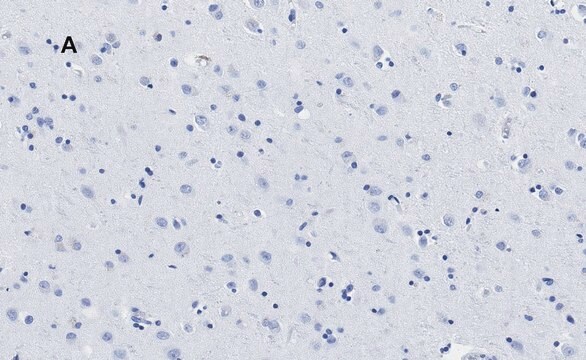
Cultured cortical neurons were immunostained for APP using rabbit anti-phospho-APP (pThr688) antibody.
Rabbit Anti-phospho-APP (pThr668) antibody can be used for western blot applications at a dilution of 1:1,000.
Physical form
Solution in Dulbecco′s phosphate buffered saline (without Mg2+ and Ca2+), pH 7.3, with 50% glycerol, 1.0 mg/mL BSA (IgG and protease free) and 0.05% sodium azide.
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Chun-Yan Wang et al.
Antioxidants & redox signaling, 30(11), 1411-1431 (2018-04-11)
Oxidative stress and neuroinflammation play important roles in the pathology of Alzheimer's disease (AD). Thioredoxin-interacting protein (TXNIP), an endogenous inhibitor of antioxidant thioredoxin, is suspected to be an important modulator of oxidative stress and inflammation. However, the underlying mechanism involved
Yoichi Ohshima et al.
The Journal of toxicological sciences, 43(4), 257-266 (2018-04-06)
The increased ratio of longer amyloid-β (Aβ1-42)/shorter amyloid-β (Aβ1-40) peptides, generated from amyloid precursor protein (APP), is known to promote the development of Alzheimer's disease (AD). To investigate the role of smoking in Aβ production, we determined the production of
Roberta Roncarati et al.
Proceedings of the National Academy of Sciences of the United States of America, 99(10), 7102-7107 (2002-05-16)
The beta-amyloid precursor protein (APP) and the Notch receptor undergo intramembranous proteolysis by the Presenilin-dependent gamma-secretase. The cleavage of APP by gamma-secretase releases amyloid-beta peptides, which have been implicated in the pathogenesis of Alzheimer's disease, and the APP intracellular domain
D Goldgaber et al.
Science (New York, N.Y.), 235(4791), 877-880 (1987-02-20)
Four clones were isolated from an adult human brain complementary DNA library with an oligonucleotide probe corresponding to the first 20 amino acids of the beta peptide of brain amyloid from Alzheimer's disease. The open reading frame of the sequenced
R E Tanzi et al.
Science (New York, N.Y.), 235(4791), 880-884 (1987-02-20)
The amyloid beta protein has been identified as an important component of both cerebrovascular amyloid and amyloid plaques of Alzheimer's disease and Down syndrome. A complementary DNA for the beta protein suggests that it derives from a larger protein expressed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service