A7236
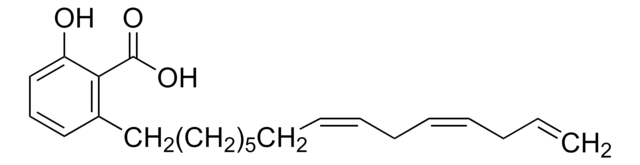
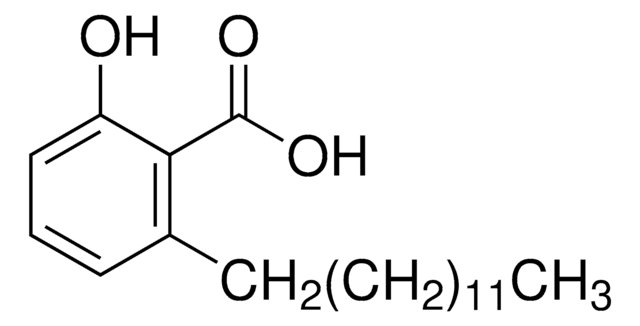
Anacardic acid
Synonym(s):
2-Hydroxy-6-pentadecylbenzoic acid, 22:0-Anacardic acid, 6-Pentadecylsalicylic acid
About This Item
Recommended Products
form
powder
Quality Level
storage condition
protect from light
color
white to beige
solubility
DMSO: ≥20 mg/mL
storage temp.
−20°C
SMILES string
CCCCCCCCCCCCCCCc1cccc(O)c1C(O)=O
InChI
1S/C22H36O3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-16-19-17-15-18-20(23)21(19)22(24)25/h15,17-18,23H,2-14,16H2,1H3,(H,24,25)
InChI key
ADFWQBGTDJIESE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- as a histone acetylase (HAT) inhibitor to study its effects on rat cortical neurons
- as a positive control in acetylation assay in vitro
- as an acetylase inhibitor to study its effects on the ribonucleic acid export 1 (Rae-1) protein acetylation that was transfected in human embryonic kidney cells
Biochem/physiol Actions
Features and Benefits
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Epigenetic modifications are thought to occur through two key interconnected processes—DNA methylation and the covalent modification of histones.
Related Content
We offer a variety of small molecule research tools, such as transcription factor modulators, inhibitors of chromatin modifying enzymes, and agonists/antagonists for target identification and validation in gene regulation research; a selection of these research tools is shown below.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service