All Photos(3)
About This Item
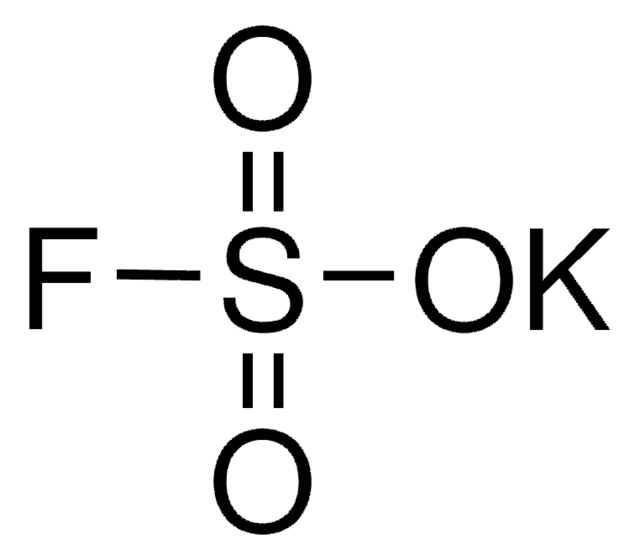
Linear Formula:
K2SO4
CAS Number:
Molecular Weight:
174.26
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.55
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
≥99.0%
form
powder
SMILES string
[K+].[K+].[O-]S([O-])(=O)=O
InChI
1S/2K.H2O4S/c;;1-5(2,3)4/h;;(H2,1,2,3,4)/q2*+1;/p-2
InChI key
OTYBMLCTZGSZBG-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
General description
Potassium sulfate is an inorganic salt that can be prepared by reacting phosphogypsum and potassium chloride. It forms needlelike mullite particles on heating with aluminum sulfate (Al2(SO4)3) and silicon dioxide (SiO2). Its application to the soil has been reported to minimize the bronzing of rice plants. Its surface integration growth kinetics has been obtained in the temperature range of 20-50°C. The theoretical heat capacity curve of potassium sulfate in vapor phase has been obtained.
Application
Potassium sulfate may be used as a sulfonating agent in the preparation of 4′-dibenzo-18-crown-6-sulfonic acid via sulfonation of dibenzo-18-crown-6.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Growth and dissolution kinetics of potassium sulfate crystals in an agitated vessel.
Garside J, et al.
Industrial & Engineering Chemistry Fundamentals, 13(4), 299-305 (1974)
Thermodynamics of the vaporization processes for potassium sulfate.
Eliezer I and Howald RA.
J. Chem. Phys., 65(8), 3053-3062 (1976)
Sulfonation of benzocrown ethers by potassium sulfate in polyphosphoric acid.
Grebenyuk AD, et al.
Chemistry of Heterocyclic Compounds, 37(7), 822-826 (2001)
Rice bronzing in Nigeria caused by nutrient imbalances and its control by potassium sulfate application.
Yamauchi M.
Plant and Soil, 117(2), 275-286 (1989)
Conversion of phosphogypsum to potassium sulfate.
Aagli A, et al.
Journal of Thermal Analysis and Calorimetry, 82(2), 395-399 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service