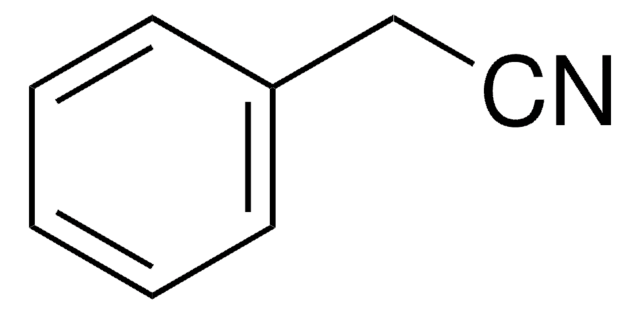
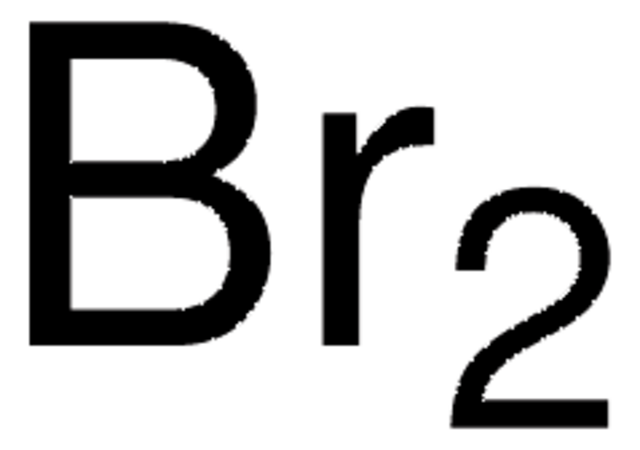B8959
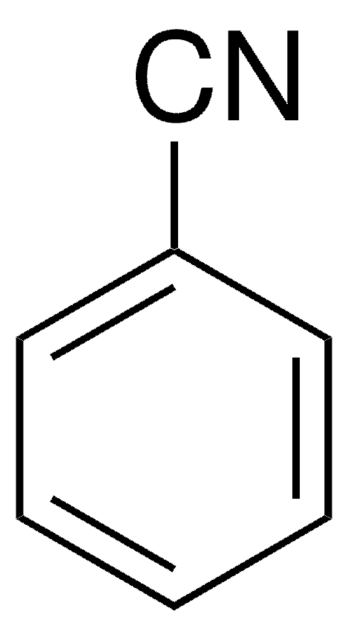
Benzonitrile
ReagentPlus®, 99%
Synonym(s):
Phenyl cyanide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CN
CAS Number:
Molecular Weight:
103.12
Beilstein:
506893
EC Number:
MDL number:
UNSPSC Code:
12352117
eCl@ss:
39031505
PubChem Substance ID:
NACRES:
NA.21
Assay:
99%
bp:
191 °C (lit.)
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
99%
form
liquid
expl. lim.
0.34-6.3 %
refractive index
n20/D 1.528 (lit.)
bp
191 °C (lit.)
mp
−13 °C (lit.)
SMILES string
N#Cc1ccccc1
InChI
1S/C7H5N/c8-6-7-4-2-1-3-5-7/h1-5H
InChI key
JFDZBHWFFUWGJE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Benzonitrile, also known as a phenyl cyanide compound, is a useful solvent and a versatile precursor to many derivatives. It is a good solvent for the study of inorganic, organic, anhydrous, and organometallic compounds.
Application
Benzonitrile can be used as:
- An electrochemical solvent to investigate the electrochemistry, spectroscopic properties, and reactivity of a series of cobalt porphyrins with various substituents.
- Building block or starting material in various organic synthesis reactions.
- Employed in coupling reactions, such as Suzuki couplings or Heck reactions, to facilitate the formation of carbon-carbon bonds.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 1
Flash Point(F)
158.0 °F - closed cup
Flash Point(C)
70 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jessica L Durham et al.
Inorganic chemistry, 51(14), 7825-7836 (2012-07-07)
The preparation of two new families of hexanuclear rhenium cluster complexes containing benzonitrile and phenyl-substituted tetrazolate ligands is described. Specifically, we report the preparation of a series of cluster complexes with the formula [Re(6)Se(8)(PEt(3))(5)L](2+) where L = benzonitrile, p-aminobenzonitrile, p-methoxybenzonitrile
Rocío López-Rodríguez et al.
The Journal of organic chemistry, 77(21), 9915-9920 (2012-10-18)
A mild procedure for the Ir(III)-catalyzed nitrogen-directed ortho borylation of aromatic N,N-dialkylhydrazones using pinacolborane as the boron source has been developed. The methodology relies on a modified, hemilabile N,N ligand built on a 4-N,N-dimethylaminopyridine unit that provides high reactivity while
Mohsen Sajadi et al.
Physical chemistry chemical physics : PCCP, 13(39), 17768-17774 (2011-09-03)
Time-dependent Stokes shifts (TDSS) were measured for diverse polarity probes in water, heavy water, methanol, and benzonitrile, by broadband fluorescence up-conversion with 85 fs time resolution. In water the spectral dynamics is solute-independent and quantitatively described by simple dielectric continuum
Yoshimitsu Hashimoto et al.
Organic & biomolecular chemistry, 10(30), 6003-6009 (2012-05-19)
A variety of highly functionalized polycyclic isoxazoles are prepared by a two-step protocol: (1) 1,3-dipolar cycloaddition of o,o'-disubstituted benzonitrile oxides to para-quinone mono-acetals, then (2) dehydrogenation. The cycloaddition proceeds in a regioselective manner, favouring the formation of the 4-acyl cycloadducts
Johanna Ungersboeck et al.
Applied radiation and isotopes : including data, instrumentation and methods for use in agriculture, industry and medicine, 70(11), 2615-2620 (2012-09-04)
The intention for the present study was to implement a microfluidic set-up for N-(11)C-methylations in a flow-through microreactor device with [(11)C]DASB as model-compound and [(11)C]CH(3)I and [(11)C]CH(3)OTf, respectively, as (11)C-methylation agents. Due to an observed "aging" effect of the (11)C-methylation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service