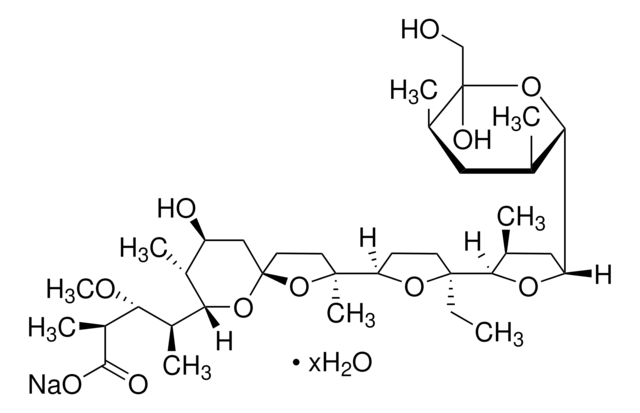
30552
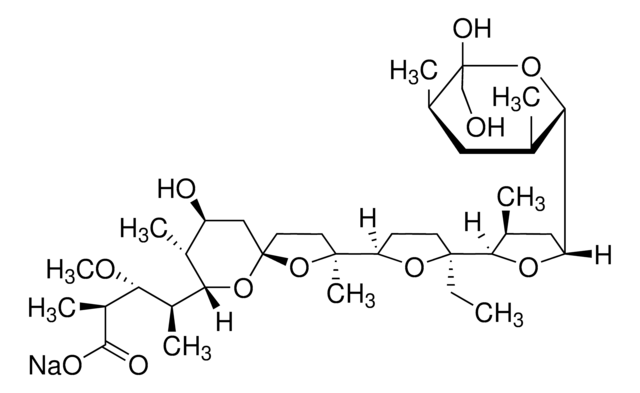
Monensin methyl ester
Selectophore™, ≥97.0% (TLC)
Synonym(s):
Methyl monensin
About This Item
Recommended Products
grade
for ion-selective electrodes
Quality Level
product line
Selectophore™
Assay
≥97.0% (TLC)
storage temp.
2-8°C
SMILES string
CC[C@]1(CCC(O1)[C@]2(C)CC[C@]3(C[C@H](O)[C@@H](C)C(O3)[C@@H](C)[C@@H](OC)[C@H](C)C(=O)OC)O2)C4OC(C[C@@H]4C)C5O[C@@](O)(CO)[C@H](C)C[C@@H]5C
InChI
1S/C37H64O11/c1-11-35(32-21(3)17-27(44-32)29-20(2)16-22(4)37(41,19-38)47-29)13-12-28(45-35)34(8)14-15-36(48-34)18-26(39)23(5)31(46-36)24(6)30(42-9)25(7)33(40)43-10/h20-32,38-39,41H,11-19H2,1-10H3/t20-,21+,22+,23+,24+,25+,26-,27-,28+,29-,30-,31-,32-,34+,35-,36+,37-/m0/s1
InChI key
PFRZSHIENRKVSE-RJTHVKINSA-N
General description
Application
- The selectivity of membrane ion-selective electrodes: This research explores the use of temperature variations to adjust the selectivity of ion-selective electrodes, utilizing Monensin methyl ester as a key component for sodium ion detection (Zahran et al., 2010).
- Spectroscopic and semiempirical studies of a proton channel formed by the methyl ester of monensin A.: This paper presents a detailed analysis of the proton channel properties of Monensin methyl ester through spectroscopic and computational methods, highlighting its potential in analytical chemistry applications (Huczyński et al., 2006).
- Ion chromatography detector based on solid-state ion-selective electrode array.: The development of an ion chromatography detector employing solid-state ion-selective electrodes, with Monensin methyl ester playing a crucial role in sodium ion detection, is detailed in this study (Lee et al., 2000).
Packaging
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service