MAB8705
Anti-Dengue Virus Complex Antibody, clone D3-2H2-9-21
clone D3-2H2-9-21, Chemicon®, from mouse
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352203
eCl@ss:
32160702
NACRES:
NA.41
Recommended Products
biological source
mouse
Quality Level
antibody form
purified immunoglobulin
clone
D3-2H2-9-21, monoclonal
species reactivity
human
manufacturer/tradename
Chemicon®
technique(s)
immunocytochemistry: suitable
immunofluorescence: suitable
isotype
IgG1
IgG2a
shipped in
wet ice
General description
Dengue fever is an acute, mosquito-transmitted viral disease characterized by fever, headache, arthralgia (severe retro-orbital pain), myalgia, rash, nausea, and vomiting. Infections are caused by any of the four closely related, but antigenically distinct virus serotypes (DEN-1, DEN-2, DEN-3, and DEN-4). Infection with one of these serotypes does not provide cross-protective immunity, so persons living in a dengue-endemic area can have four dengue infections during their lifetimes. Dengue is primarily an urban disease of the tropics, and the viruses that cause it are maintained in a cycle that involves humans and Aedes aegypti, a domestic, day-biting mosquito that prefers to feed on humans. Although most dengue infections result in relatively mild illness, some can produce Dengue Hemorrhagic Fever (DHF) or dengue shock syndrome, with children being particularly at risk. Although epidemic outbreaks have been reported since 1779, the incidence has been increasing, with global, multiple serotype pandemics intensifying within the last 15 years. There is no specific antiviral therapy for dengue, but for both classical dengue and dengue hemorrhagic fever, symptomatic and supportive measures are effective. Important risk factors for DHF include the strain and serotype of the virus involved, as well as the age, immune status, and genetic predisposition of the patient.
Specificity
Reacts with all members of the Dengue complex.
Immunogen
Dengue type 4 virus antigens.
Application
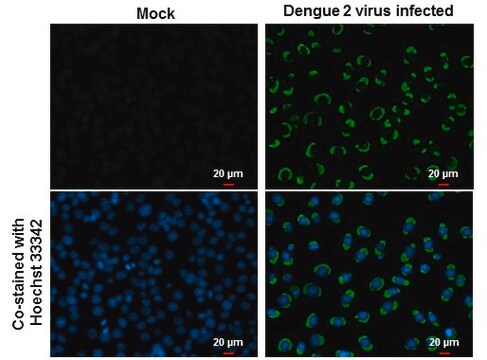
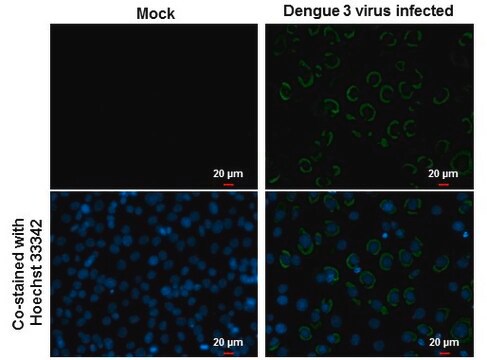
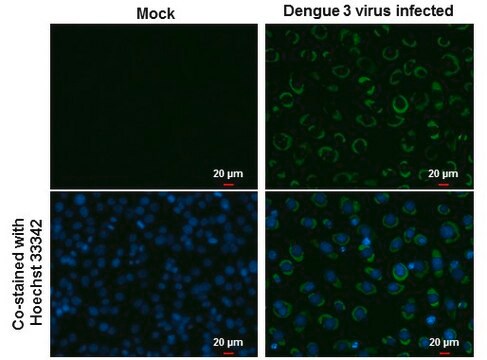
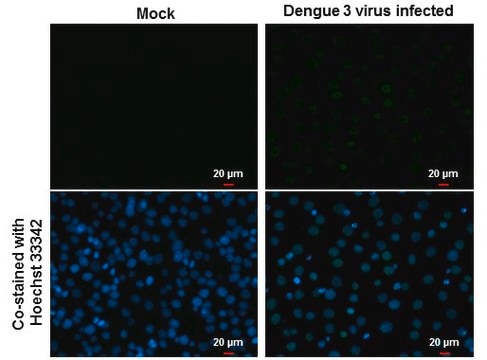
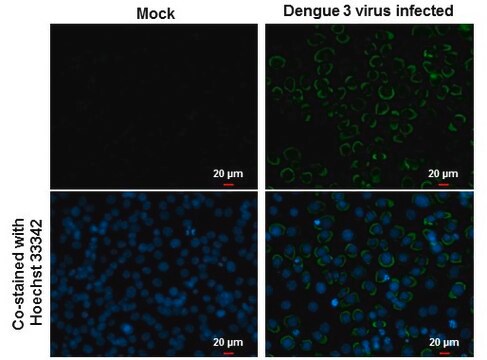
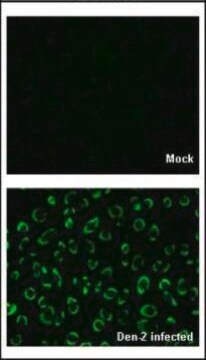
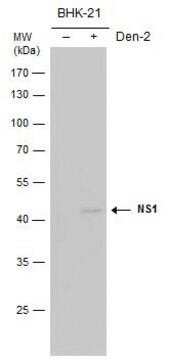
Detect Dengue Virus Complex using this Anti-Dengue Virus Complex Antibody, clone D3-2H2-9-21 validated for use in IC & IF.
Immunofluorescence.
Immunocytochemistry on acetone fixed material.
Suggested working dilution: 1:50-400.
Final working dilutions must be determined by end user.
Immunocytochemistry on acetone fixed material.
Suggested working dilution: 1:50-400.
Final working dilutions must be determined by end user.
Research Category
Infectious Diseases
Infectious Diseases
Research Sub Category
Infectious Diseases - Viral
Infectious Diseases - Viral
Physical form
Format: Purified
Protein A Purified mouse immunoglobulin in 20 mM sodium phosphate, 250 mM NaCl, pH. 7.6, with 0.1% sodium azide as a preservative.
Protein A purified
Storage and Stability
Maintain for 6 months at 2–8°C from date of shipment. Aliquot to avoid repeated freezing and thawing. For maximum recovery of product, centrifuge the original vial after thawing and prior to removing the cap.
Analysis Note
Control
Dengue positive patient sample
Dengue positive patient sample
Other Notes
Concentration: Please refer to the Certificate of Analysis for the lot-specific concentration.
Legal Information
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Albin Fontaine et al.
Scientific reports, 6, 24885-24885 (2016-04-28)
Successful transmission of a vector-borne pathogen relies on a complex life cycle in the arthropod vector that requires initial infection of the digestive tract followed by systemic viral dissemination. The time interval between acquisition and subsequent transmission of the pathogen
Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention.
Goncalvez, AP; Engle, RE; St Claire, M; Purcell, RH; Lai, CJ
Proceedings of the National Academy of Sciences of the USA null
Christiane Fernandes Ribeiro et al.
International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases, 55, 109-112 (2017-01-16)
A histopathological and immunohistochemical study was conducted in placental tissues and retained products of conception from 24 patients with confirmed dengue infection during pregnancy. The immunohistochemical assay was positive for dengue virus in 19 placental and three ovular remnants analyzed.
Nattapol Attatippaholkun et al.
Scientific reports, 8(1), 2688-2688 (2018-02-11)
Since the hemorrhage in severe dengue seems to be primarily related to the defect of the platelet, the possibility that dengue virus (DENV) is selectively tropic for one of its surface receptors was investigated. Flow cytometric data of DENV-infected megakaryocytic
Yun Young Go et al.
Journal of clinical microbiology, 54(6), 1528-1535 (2016-04-01)
Dengue virus (DENV) infection is considered a major public health problem in developing tropical countries where the virus is endemic and continues to cause major disease outbreaks every year. Here, we describe the development of a novel, inexpensive, and user-friendly
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service