W530597
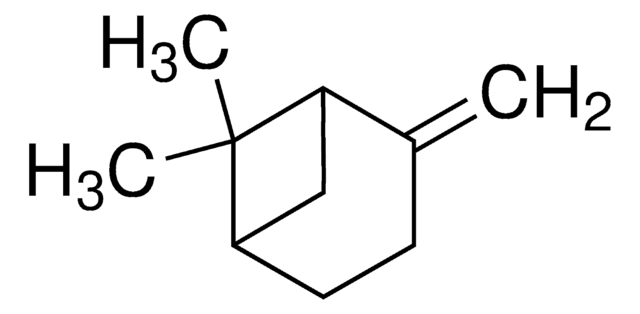
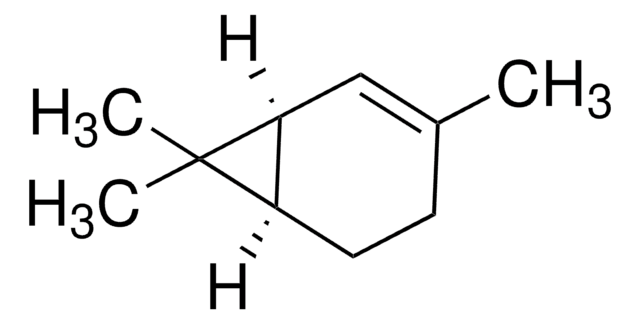
Sabinene
natural, 75%
Synonym(s):
4(10)-thujene, Sabinen, 4-methylidene-1-propan-2-ylbicyclo[3.1.0]hexane
About This Item
Recommended Products
grade
Fragrance grade
Halal
Kosher
natural
Agency
follows IFRA guidelines
reg. compliance
EU Regulation 1223/2009
Assay
75%
greener alternative product characteristics
Less Hazardous Chemical Syntheses
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
density
0.842 g/mL at 25 °C
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
fragrance allergen
no known allergens
greener alternative category
Organoleptic
woody; citrus; spicy
SMILES string
CC(C)C12CCC(=C)C1C2
InChI
1S/C10H16/c1-7(2)10-5-4-8(3)9(10)6-10/h7,9H,3-6H2,1-2H3
InChI key
NDVASEGYNIMXJL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Disclaimer
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
-Cymene; Linalool; Menthol; α-Terpineol; Menthyl acetate
-α-Bergamotene; β-Bisabolene; α-Terpineol; Neryl acetate; Geranyl acetate; Neral; Geranial
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service