T45802
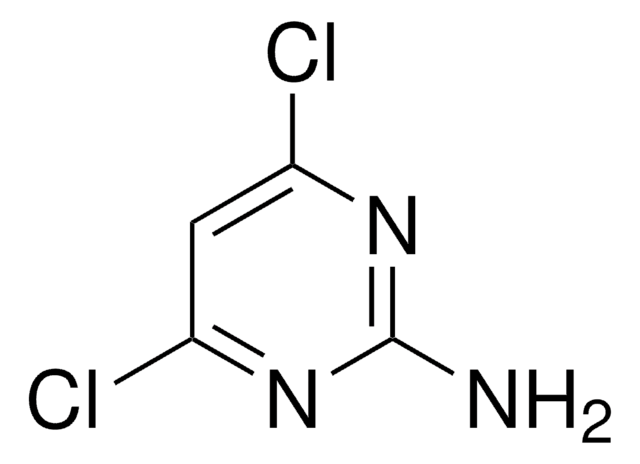
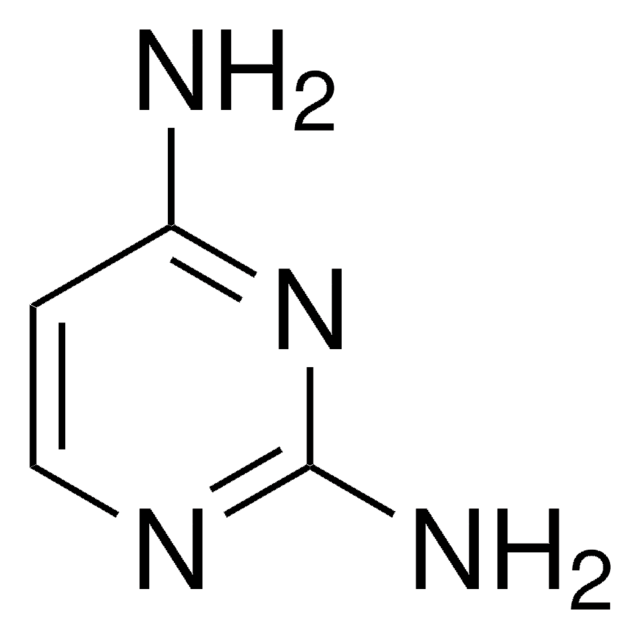
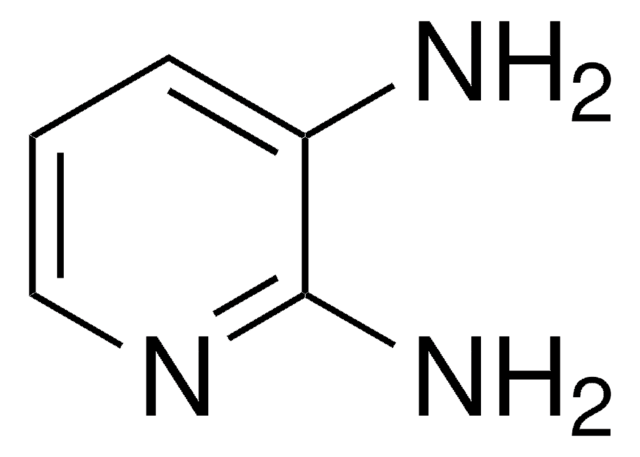
2,4,6-Triaminopyrimidine
97%
Synonym(s):
2,4,6-Pyrimidinetriamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H7N5
CAS Number:
Molecular Weight:
125.13
Beilstein:
118448
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
249-251 °C (lit.)
SMILES string
Nc1cc(N)nc(N)n1
InChI
1S/C4H7N5/c5-2-1-3(6)9-4(7)8-2/h1H,(H6,5,6,7,8,9)
InChI key
JTTIOYHBNXDJOD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
W L Armarego et al.
The Biochemical journal, 211(2), 357-361 (1983-05-01)
The Km and kcat. values for [6,6,7,7-2H]7,8(6H)-dihydropterin and 2,6-diamino-5-iminopyrimidin-4-one were determined for dihydropteridine reductase (EC 1.6.99.10) from two sources. The parameters of the pterin are of the same order as those of the most effective substrates of dihydropteridine reductase. The
S D Provan et al.
The Journal of pharmacology and experimental therapeutics, 245(3), 928-931 (1988-06-01)
An in situ rat gut preparation was used to elucidate the mechanisms of gastrointestinal aluminum (Al) absorption. Al uptake rate at the mucosal surface was decreased by the paracellular pathway blockers kinetin (1 mM) and 2,4,6-triaminopyrimidinium (10 mM), by sodium
M P Vinardell et al.
Revista espanola de fisiologia, 39(2), 193-196 (1983-06-01)
D-glucose diffusion in both jejunum and ileum using a perfusion system in vivo was determined. 2,4,6-triaminopyrimidine (20 mM) induced an inhibition on D-glucose diffusion of 32% in the two segments of the small intestine studied. Glucose net efflux from the
R Soni et al.
Journal of the National Cancer Institute, 93(6), 436-446 (2001-03-22)
Cyclin-dependent kinase 4 (Cdk4) represents a prime target for the treatment of cancer because most human cancers are characterized by overexpression of its activating partner cyclin D1, loss of the natural Cdk4-specific inhibitor p16, or mutation(s) in Cdk4's catalytic subunit.
Chris M Wood et al.
The Journal of experimental biology, 215(Pt 3), 508-517 (2012-01-17)
Paracellular permeability and absorptive water flux across the intestine of the euryhaline killifish were investigated using in vitro gut sac preparations from seawater- and freshwater-acclimated animals. The permeability of polyethylene glycol (PEG), a well-established paracellular probe, was measured using trace
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service