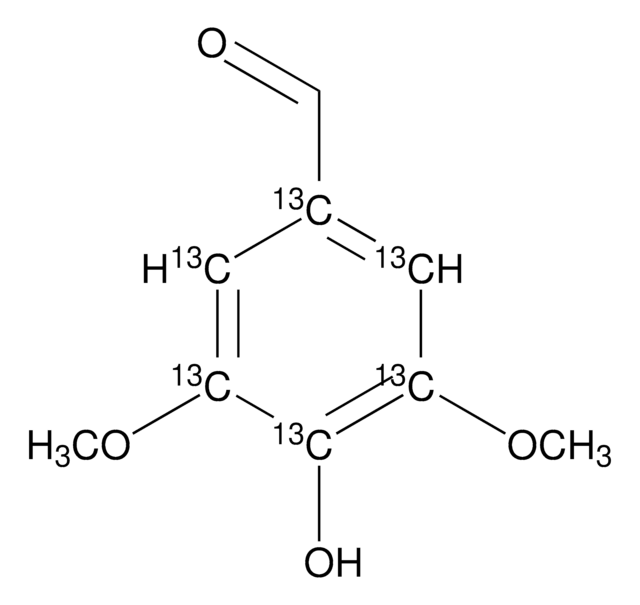
S7602
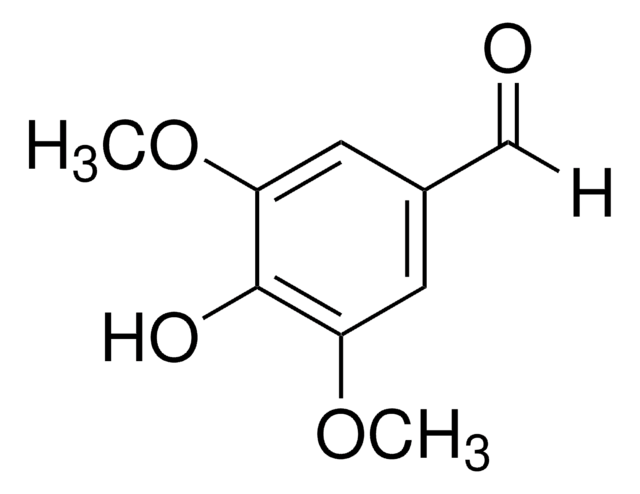
Syringaldehyde
98%
Synonym(s):
3,5-Dimethoxy-4-hydroxybenzaldehyde, 4-Hydroxy-3,5-dimethoxybenzaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
HOC6H2(OCH3)2CHO
CAS Number:
Molecular Weight:
182.17
Beilstein:
784514
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
bp
192-193 °C/14 mmHg (lit.)
mp
110-113 °C (lit.)
SMILES string
COc1cc(C=O)cc(OC)c1O
InChI
1S/C9H10O4/c1-12-7-3-6(5-10)4-8(13-2)9(7)11/h3-5,11H,1-2H3
InChI key
KCDXJAYRVLXPFO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cuimin Hu et al.
Bioresource technology, 100(20), 4843-4847 (2009-06-06)
Lignocellulosic biomass hydrolysis inevitably coproduces byproducts that may have various affects on downstream biotransformation. It is imperative to document the inhibitor tolerance ability of microbial strain in order to utilize biomass hydrolysate more effectively. To achieve better lipid production by
Palwinder Singh et al.
Journal of medicinal chemistry, 55(14), 6381-6390 (2012-06-28)
On the basis of structural analysis of dihydrofolate reductase (DHFR) (cocrystallized separately with NADPH, dihydrofolate and NADPH, trimethoprim), compounds 2 and 3 were optimized for inhibition of DHFR. Appreciable tumor growth inhibitory activities of compounds 2 and 3 over 60
Chia-Hsin Huang et al.
Journal of natural products, 75(8), 1465-1468 (2012-08-14)
The antihyperglycemic effect of syringaldehyde (1), purified from the stems of Hibiscus taiwanensis, was investigated in streptozotocin-induced diabetic rats (STZ-diabetic rats) showing type-1 like diabetes mellitus. Bolus intravenous injection of 1 showed antihyperglycemic activity in a dose-dependent manner in STZ-diabetic
Rogério S Pereira et al.
Journal of industrial microbiology & biotechnology, 38(1), 71-78 (2010-09-08)
The inhibitory action of acetic acid, ferulic acid, and syringaldehyde on metabolism of Candida guilliermondii yeast during xylose to xylitol bioconversion was evaluated. Assays were performed in buffered and nonbuffered semidefined medium containing xylose as main sugar (80.0 g/l), supplemented
Laura Mendoza et al.
Enzyme and microbial technology, 49(5), 478-484 (2011-11-25)
This paper presents the use of a membrane-integrated reactor system with recycling of laccase and mediator for azo dye decolorization. From initial screening of different laccases and mediators, Trametes versicolor laccase and syringaldehyde provided the best system for decolorization. Decolorization
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![n-Amyl 2-[3,5-dihydroxy-2-(1-nonanoyl)phenyl]acetate ≥98% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/350/895/d005c065-901e-47b0-b409-26ccd1eba4d1/640/d005c065-901e-47b0-b409-26ccd1eba4d1.png)