P45605
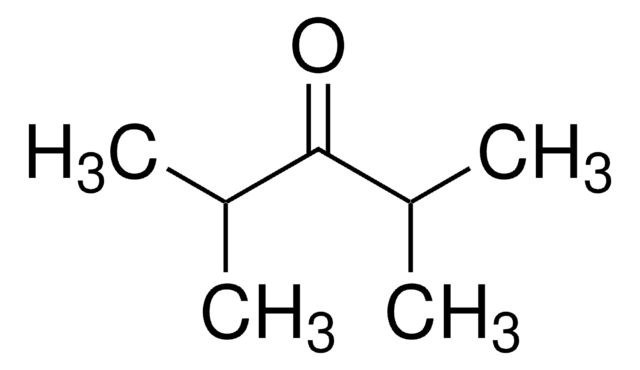
3,3-Dimethyl-2-butanone
97%
Synonym(s):
α,α,α-Trimethylacetone, tert-Butyl methyl ketone, Pinacolone
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3COC(CH3)3
CAS Number:
Molecular Weight:
100.16
Beilstein:
1209331
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.396 (lit.)
bp
106 °C (lit.)
density
0.801 g/mL at 25 °C (lit.)
SMILES string
CC(=O)C(C)(C)C
InChI
1S/C6H12O/c1-5(7)6(2,3)4/h1-4H3
InChI key
PJGSXYOJTGTZAV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
3,3-Dimethyl-2-butanone is an aliphatic ketone can undergo asymmetric reduction to the corresponding alcohol with diisopinocampheylchloroborane with high enantiomeric excess.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
41.0 °F - closed cup
Flash Point(C)
5 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Keliang Gao et al.
Applied microbiology and biotechnology, 71(6), 819-823 (2006-02-21)
Enantioselective biotransformation of DL-1,2-propanediol to D-2-hydroxypropanic acid was first reported by the authors. In the biooxidation process, there were some by-product formed and thus influenced the e.e. value and output of the acid. Restricting oxygen in the reaction system and
Y Chen et al.
The Journal of organic chemistry, 66(11), 3930-3939 (2001-05-26)
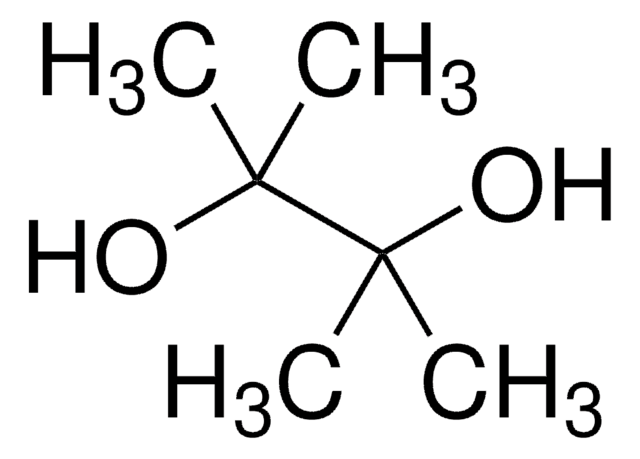
To investigate the effects of electron-donating and electron-withdrawing substituents upon the reaction of porphyrins with osmium tetraoxide, and the pinacol-pinacolone rearrangement of the resulting diols, a series of meso-substituted porphyrins were prepared by total synthesis. Porphyrins with electron-donating substitutents at
Makoto Shimizu et al.
Organic letters, 4(23), 4097-4099 (2002-11-09)
The pinacol reaction of beta-halogenated alpha,beta-unsaturated aldehydes was promoted by titanium tetraiodide to give coupling products in good yields with high dl-selectivity. Subsequent reduction with H(2)/Pd-C gave saturated vic-diols in good yields. Heck coupling reaction enabled the displacement of halogens
Direct conversion of arylamines to pinacol boronates: a metal-free borylation process.
Fanyang Mo et al.
Angewandte Chemie (International ed. in English), 49(10), 1846-1849 (2010-02-04)
Richard E Mishler et al.
Chemical communications (Cambridge, England), (41)(41), 6201-6203 (2009-10-15)
We report a new and simple one-pot synthetic method to produce mesoporous silica and nanoporous solid acid catalyst capable of catalyzing pinacole-pinacolone rearrangement and esterification reactions, by preparing a solvent washable phosphonated triblock copolymer template and self-assembling it in the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service