N257
Naphthalene-1-boronic acid
≥95.0%
Synonym(s):
α-Naphthaleneboronic acid, α-Naphthylboronic acid, 1-Naphthaleneboronic acid, 1-Naphthylboronic acid, 1-naphthalenyl-boronic acid, NSC 78936
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C10H7B(OH)2
CAS Number:
Molecular Weight:
171.99
Beilstein:
2937504
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0%
form
crystals
mp
208-214 °C (lit.)
SMILES string
OB(O)c1cccc2ccccc12
InChI
1S/C10H9BO2/c12-11(13)10-7-3-5-8-4-1-2-6-9(8)10/h1-7,12-13H
InChI key
HUMMCEUVDBVXTQ-UHFFFAOYSA-N
Related Categories
Application
Naphthalene-1-boronic acid can be used:
- Methyl 5-bromo-2-(naphthalene-1-yl)benzoate, which is a starting material for the preparation of 9-anthracene-spirobenzofluorene host materials.
- A bifunctional polymer used for the hydrolysis of cellulose.
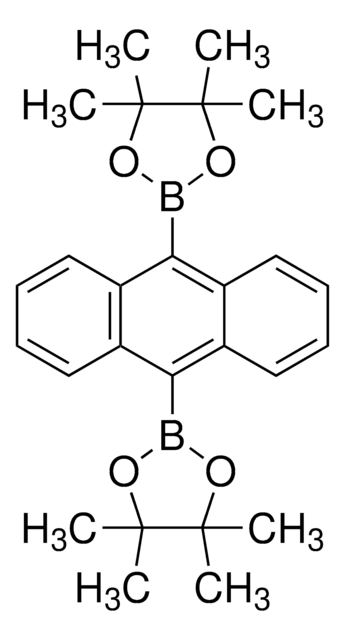
- 9-(Naphthalene-1-yl)anthracene, which is a key intermediate for the preparation of anthracene derived molecular glass having blue emission.
Other Notes
Contains varying amounts of anhydride
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Bifunctional porous polymers bearing boronic and sulfonic acids for hydrolysis of cellulose
Yang Q and Pan X
ACS sustainable chemistry & engineering, 4(9), 4824-4830 (2016)
Suzuki homo-coupling reaction based fluorescent sensors for monosaccharides
Xu S, et al.
Royal Society of Chemistry Advances, 4(66), 35238-35241 (2014)
Highly efficient blue OLED based on 9-anthracene-spirobenzofluorene derivatives as host materials
Gong M, et al.
Journal of Materials Chemistry, 20(47), 10735-10746 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service