All Photos(2)
About This Item
Empirical Formula (Hill Notation):
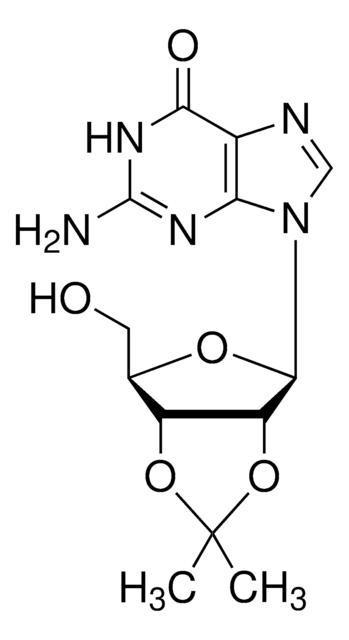
C13H17N5O4
CAS Number:
Molecular Weight:
307.31
Beilstein:
43435
EC Number:
MDL number:
UNSPSC Code:
12352204
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
optical activity
[α]20/D −98.5°, c = 1 in dioxane
mp
221-222 °C (lit.)
SMILES string
CC1(C)O[C@@H]2[C@@H](CO)O[C@H]([C@@H]2O1)n3cnc4c(N)ncnc34
InChI
1S/C13H17N5O4/c1-13(2)21-8-6(3-19)20-12(9(8)22-13)18-5-17-7-10(14)15-4-16-11(7)18/h4-6,8-9,12,19H,3H2,1-2H3,(H2,14,15,16)/t6-,8-,9-,12-/m1/s1
InChI key
LCCLUOXEZAHUNS-WOUKDFQISA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M Brignoni et al.
Journal of cell science, 108 ( Pt 5), 1931-1943 (1995-05-01)
Madin-Darby canine kidney and other epithelial cell lines (e.g. Caco-2, MCF-10A and MCF-7) develop intracellular vacuoles composed of apical membrane displaying microvilli (VACs) when impaired from forming normal cell-to-cell contacts. In a previous publication, we showed that VACs are rapidly
I D Golovatskiĭ et al.
Ukrainskii biokhimicheskii zhurnal (1978), 58(3), 37-40 (1986-05-01)
Transformation of synthesized 2',3'-O-isopropylidene adenosine was studied in comparison with adenosine in rat liver homogenates. It is stated that 2',3'-O-isopropylidene adenosine is subjected to deamination similar to adenosine but less intensively. Due to deamination 2',3'-O-isopropylidene inosine is formed from 2',3'-O-isopropylidene
Pierangela Ciuffreda et al.
Nucleosides, nucleotides & nucleic acids, 26(10-12), 1311-1313 (2007-12-11)
2 ',3 '-Isopropylidene group can be used as a molecular scaffold for the introduction of modifications at 5 ' and 1 ' positions of adenosine and these modified nucleosides are used to evaluate the biocatalytic activity of adenosine and adenylate
Lindsay R Comstock et al.
The Journal of organic chemistry, 69(4), 1425-1428 (2004-02-14)
8-Azido-5'-aziridino-5'-deoxyadenosine (6), a novel cofactor mimic, was synthesized in nine steps from commercially available 2',3'-isopropylideneadenosine in approximately 4% overall yield. Crucial to this success was a very unorthodox phthalimide cleavage procedure, C8 azidation prior to aziridination and late stage alkylation
I D Golovatskiĭ et al.
Ukrainskii biokhimicheskii zhurnal (1978), 61(2), 64-69 (1989-03-01)
Transformation and uptake of [8-14C]-adenosine and its synthetic analog 2',3'-O-isopropylideneadenosine was studied in Zajdel hepatoma cells and their homogenates. Uptake and deamination of adenosine and 2',3'-O-isopropylideneadenosine by Zajdel hepatoma cells proceed differently. A small part of adenosine is phosphorylated and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service