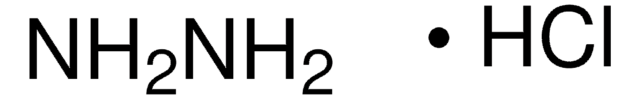751855
Hydrazine solution
1 M in acetonitrile
Synonym(s):
Hydrazine, Nitrogen hydride
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
NH2NH2
CAS Number:
Molecular Weight:
32.05
Beilstein:
878137
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
liquid
Quality Level
concentration
1 M in acetonitrile
refractive index
n20/D 1.348
density
0.779 g/mL at 25 °C
SMILES string
NN
InChI
1S/H4N2/c1-2/h1-2H2
InChI key
OAKJQQAXSVQMHS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Hydrazine (N2H4) solution is a common reducing agent and a versatile reagent in organic synthesis. It can reduce a variety of functional groups like ketones, aldehydes, imines, nitro compounds, azides & nitrates to their corresponding amines. It also serves as a source of nitrogen in the synthesis of heterocyclic compounds, such as pyrazoles and pyrazolines.
Application
Hydrazine solution (in acetonitrile) can be used as a reducing source for the reduction of carbon-carbon multiple bonds in alkenes, alkynes, and α,β-unsaturated esters using metal-organic frameworks (MOFs) as heterogeneous catalysts. It is also used in the synthesis of polysubstituted 2-aminoimidazoles from 2-aminopyrimidines and α-bromocarbonyl compounds.
related product
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Chronic 2 - Carc. 1B - Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
41.0 °F
Flash Point(C)
5 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
D P Elder et al.
Journal of pharmaceutical and biomedical analysis, 54(5), 900-910 (2010-12-15)
This is the latest of a series of reviews focused on the analysis of genotoxic impurities. This review summarises the analytical approaches reported in the literature relating to hydrazine, hydrazines, hydrazides and hydrazones. It is intended to provide guidance for
Min Yuan et al.
Dalton transactions (Cambridge, England : 2003), (31)(31), 6078-6088 (2010-05-08)
A combination of unique solvent properties of hydrazine enables the direct dissolution of a range of metal chalcogenides at ambient temperature, rendering this an extraordinarily simple and soft synthetic approach to prepare new metal chalcogenide-based materials. The extended metal chalcogenide
M Vogel et al.
Fresenius' journal of analytical chemistry, 366(8), 781-791 (2001-03-03)
Hydrazine reagents are a well-known group of derivatizing agents for the determination of aldehydes and ketones in liquid and gaseous samples. Within this article, the most important hydrazine reagents are critically summarized, and their major applications in different fields, including
Nilay Hazari
Chemical Society reviews, 39(11), 4044-4056 (2010-06-24)
One of the most challenging problems in small molecule activation is the development of a homogeneous catalyst for converting dinitrogen into ammonia at ambient temperatures and atmospheric pressure. A catalytic cycle based on molybdenum that converts dinitrogen into ammonia has
Tino Wilson Sanchez et al.
Bioorganic & medicinal chemistry, 21(4), 957-963 (2013-01-12)
Human lens epithelium-derived growth factor (LEDGF)/p75 plays an important role in the HIV life cycle by stimulating integrase (IN)-led viral DNA integration into cellular chromosomes. Mechanistic studies show the majority of IN inhibitors chelate magnesium ions in the catalytic active
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service