All Photos(1)
About This Item
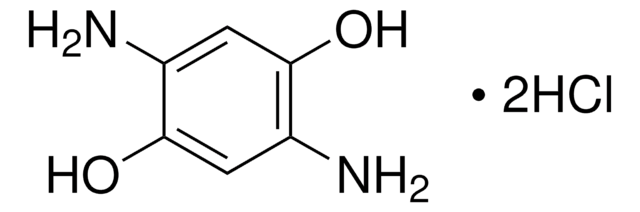
Linear Formula:
(H2N)2C6H2-1,3-(OH)2 · 2HCl
CAS Number:
Molecular Weight:
213.06
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
254 °C (dec.) (lit.)
storage temp.
2-8°C
SMILES string
Cl.Cl.Nc1cc(N)c(O)cc1O
InChI
1S/C6H8N2O2.2ClH/c7-3-1-4(8)6(10)2-5(3)9;;/h1-2,9-10H,7-8H2;2*1H
InChI key
KUMOYHHELWKOCB-UHFFFAOYSA-N
Related Categories
Application
4,6-Diaminoresorcinol dihydrochloride may be used as a precursor in the synthesis of:
- poly(p-phenylene benzobisoxazole) (PBO)
- poly(2,6-naphthalenebenzobisoxazole) (Naph-2,6-PBO)
- poly(1,5-naphthalenebenzobisoxazole) (Naph-1,5-PBO)
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Facile sulfur-assisted carbonylation of diaminoresorcinol with carbon monoxide.
Mizuno T, et al.
Heteroatom Chem., 23(1), 111-116 (2012)
A safe cost-efficient synthesis of 4,6-diaminoresorcinol.
Pews RG, et al.
The Journal of Organic Chemistry, 62(23), 8255-8256 (1997)
Synthesis, structure and properties of carbon nanotube/poly(p-phenylene benzobisoxazole) composite fibres.
Li J, et al.
Polymer International, 55(4), 456-465 (2006)
The synthesis, characterization, and crystal structures of poly (2,6-naphthalenebenzobisoxazole) and poly (1,5-naphthalenebenzobisoxazole).
Park SY, et al.
Journal of Polymer Science. Part B, Polymer Physics, 44(14), 1948-1957 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service