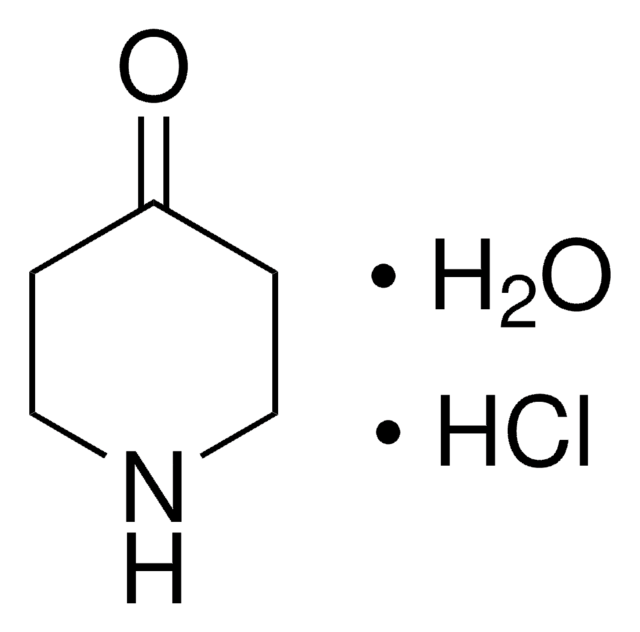
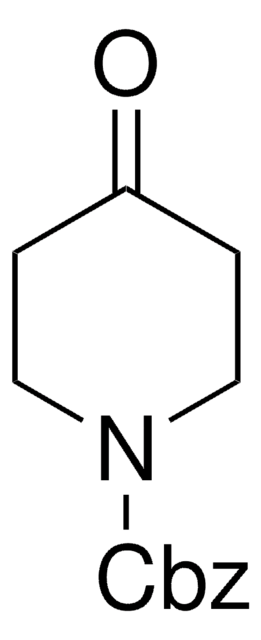
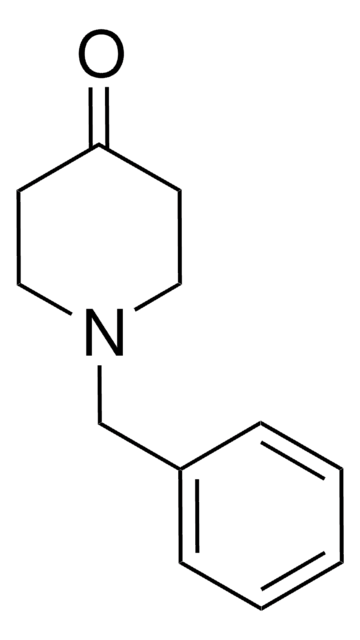
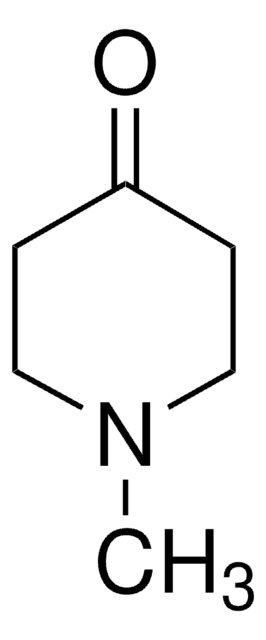
461350
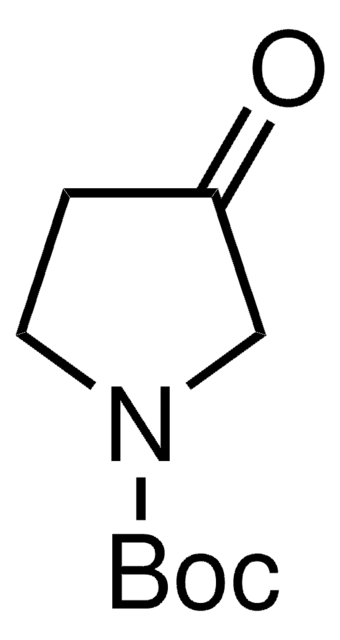
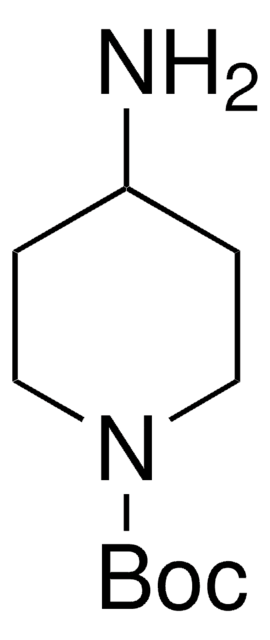
1-Boc-4-piperidone
98%
Synonym(s):
N-Boc-4-piperidone, tert-Butyl 4-oxo-1-piperidinecarboxylate
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C10H17NO3
CAS Number:
Molecular Weight:
199.25
Beilstein:
3650236
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
73-77 °C (lit.)
functional group
ketone
SMILES string
CC(C)(C)OC(=O)N1CCC(=O)CC1
InChI
1S/C10H17NO3/c1-10(2,3)14-9(13)11-6-4-8(12)5-7-11/h4-7H2,1-3H3
InChI key
ROUYFJUVMYHXFJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1-Boc-4-piperidone is used as a precursor in the synthesis of synthetic drugs and as a building block for the preparation of selective ligands.
Application
1-Boc-4-piperidone, a pharma building block, may be used in the synthesis of (3E,5E)-3,5-bis(2,5-dimethoxybenzylidene)-1-t-butoxycarbonylpiperidin-4-one (RL197). It may also be used in the synthesis of spirorifamycins containing a piperidine ring structure.
Warning
Avoid metal contact.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
In Ho Kim et al.
Bioorganic & medicinal chemistry letters, 17(5), 1181-1184 (2006-12-27)
A novel series of spirorifamycins was synthesized and their antibacterial activity evaluated both in vitro and in vivo. This new series of rifamycins shows excellent activity against Staphylococcus aureus that is equivalent to rifabutin. However, some compounds of the series
Atsuya Takami et al.
Bioorganic & medicinal chemistry, 12(9), 2115-2137 (2004-04-15)
Several structurally unrelated scaffolds of the Rho kinase inhibitor were designed using pharmacophore information obtained from the results of a high-throughput screening and structural information from a homology model of Rho kinase. A docking simulation using the ligand-binding pocket of
John W Clader et al.
Bioorganic & medicinal chemistry, 12(2), 319-326 (2004-01-16)
Anthranilamide analogues such as 23 are potent and highly selective muscarinic M2 antagonists that also show good oral bioavailability and in vivo activity.
Marlon Cowart et al.
Journal of medicinal chemistry, 47(15), 3853-3864 (2004-07-09)
A new class of agents with potential utility for the treatment of erectile dysfunction has been discovered, guided by the hypothesis that selective D4 agonists are erectogenic but devoid of the side effects typically associated with dopaminergic agents. The lead
Gebhard Thoma et al.
Journal of medicinal chemistry, 47(8), 1939-1955 (2004-04-02)
The chemokine receptor CCR5 plays an important role in inflammatory and autoimmune disorders as well as in transplant rejection by affecting the trafficking of effector T cells and monocytes to diseased tissues. Antagonists of CCR5 are believed to be of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service