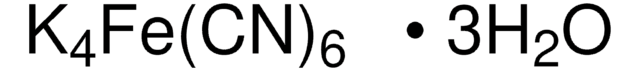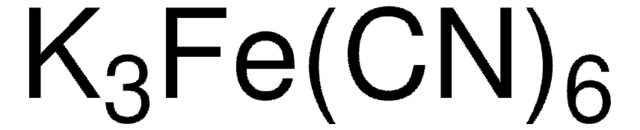455946
Potassium hexacyanoferrate(III)
99.98% trace metals basis
Synonym(s):
Potassium ferricyanide, Red prussiate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
K3Fe(CN)6
CAS Number:
Molecular Weight:
329.24
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
99.98% trace metals basis
form
crystalline
impurities
≤250.0 ppm Trace Metal Analysis
SMILES string
[K+].[K+].[K+].N#C[Fe-3](C#N)(C#N)(C#N)(C#N)C#N
InChI
1S/6CN.Fe.3K/c6*1-2;;;;/q;;;;;;-3;3*+1
InChI key
MIMJFNVDBPUTPB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Potassium ferricyanide dopant may be used to co-dope Li4Ti5O12 (LTO) with K and Fe to enhance its electrochemical performance during the use of LTO as an anode for Li-ion batteries.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Eye Irrit. 2
Supplementary Hazards
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Using potassium ferricyanide as a dopant to prepare K and Fe co-doped Li4Ti5O12
Ding K, et al.
Ceramics International, 42.16, 19187-19194 (2016)
Effects of cathodic electron acceptors and potassium ferricyanide concentrations on the performance of microbial fuel cell
Wei L, et al.
International Journal of Hydrogen Energy, 37.17, 12980-12986 (2012)
Holger Schulze et al.
Sensors (Basel, Switzerland), 21(5) (2021-04-04)
Rapid point of care tests for bacterial infection diagnosis are of great importance to reduce the misuse of antibiotics and burden of antimicrobial resistance. Here, we have successfully combined a new class of non-biological binder molecules with electrochemical impedance spectroscopy
Sveinung Lillehaug et al.
Scientific data, 6, 190028-190028 (2019-02-27)
The spatial pattern of transgene expression in tetracycline-controlled mouse models is governed primarily by the driver line used to introduce the tetracycline-controlled transactivator (tTA). Detailed maps showing where each tTA driver activates expression are therefore essential for designing and using
Hideo Takakusa et al.
Drug metabolism and disposition: the biological fate of chemicals, 39(6), 1022-1030 (2011-03-03)
Lapatinib, an oral breast cancer drug, has recently been reported to be a mechanism-based inactivator of cytochrome P450 (P450) 3A4 and also an idiosyncratic hepatotoxicant. It was suggested that formation of a reactive quinoneimine metabolite was involved in mechanism-based inactivation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service