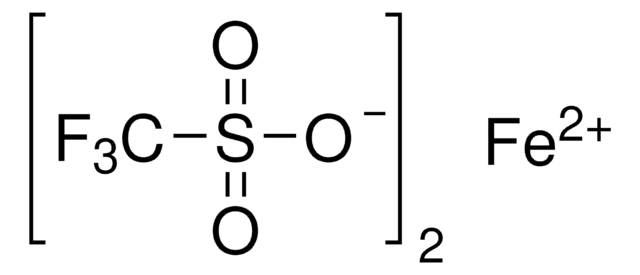
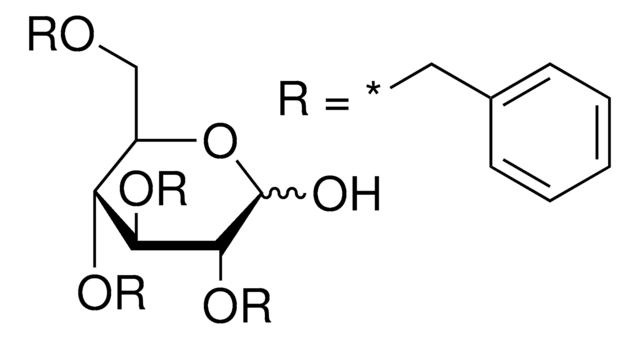
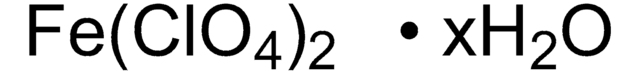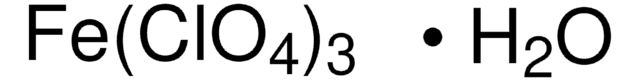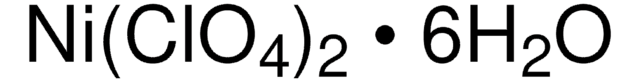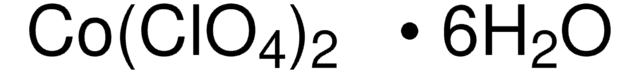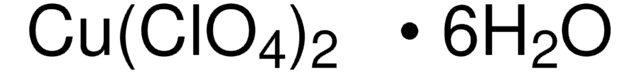309281
Iron(III) perchlorate hydrate
crystalline
Synonym(s):
Perchloric acid iron(III) salt hydrate
About This Item
Recommended Products
form
crystalline
Quality Level
reaction suitability
reagent type: oxidant
impurities
<0.10% chloride
color
yellow
SMILES string
[Fe+3].[H]O[H].[O-]Cl(=O)(=O)=O.[O-]Cl(=O)(=O)=O.[O-]Cl(=O)(=O)=O
InChI
1S/3ClHO4.Fe.H2O/c3*2-1(3,4)5;;/h3*(H,2,3,4,5);;1H2/q;;;+3;/p-3
InChI key
PFPIMAZLJXJVAN-UHFFFAOYSA-K
Looking for similar products? Visit Product Comparison Guide
Application
- Fullerene-fused lactones
- α-Carbonyl furans via one-pot cyclization
- Fullerodioxolanes via heterocyclization
- Dialylated indoles via double alkylation
- Aryl esters via oxidative esterification
Used as chemical actinometer based on photolysis of ferrioxalate in presence of polyoxometalate in aqueous solution
Oxidant for conducting polymer nanoparticles synthesized in ionic liquid by chemical polymerization
It can be used as a catalyst for synthesis of:
- Fullerene-fused lactones
- α-Carbonyl furans via one-pot cyclization
- Fullerodioxolanes via heterocyclization
- Fulleroxazolidines
- Dialylated indoles via double alkylation
- Aryl esters via oxidative esterification
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Ox. Sol. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
5.1A - Strongly oxidizing hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service