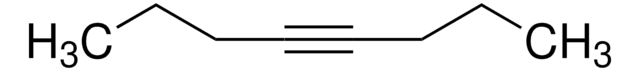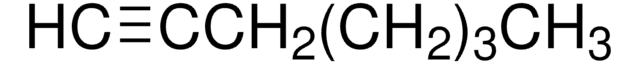All Photos(1)
About This Item
Linear Formula:
C2H5C≡CC2H5
CAS Number:
Molecular Weight:
82.14
Beilstein:
1731158
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
186 mmHg ( 37.7 °C)
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.411 (lit.)
bp
81-82 °C (lit.)
density
0.723 g/mL at 25 °C (lit.)
SMILES string
CCC#CCC
InChI
1S/C6H10/c1-3-5-6-4-2/h3-4H2,1-2H3
InChI key
DQQNMIPXXNPGCV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3-Hexyne was used in the synthesis of fused hetero-hydropyridyl ligands bonded to the {Ru(p-cymene)} organometallic moiety by reacting with ruthenacycles. It was also used in the preparation of [4+2] cycloaddition product by reacting with borole.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1 - Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
6.8 °F - closed cup
Flash Point(C)
-14 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Luciano Cuesta et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(47), 15178-15189 (2012-09-29)
Novel cycloruthenated complexes 2 a-c, 4 a-c, and 6 a, b based on heteroaromatic cores have been synthesized by reaction of a series of heterocycle-based imines with [{RuCl(η(6)-p-cymene)}(2)(μ-Cl)(2)] and Cu(OAc)(2). This approach has proved efficient for the cyclometalation of thiophene, benzothiophene, furan, benzofuran, pyrrole
Fang Ge et al.
Journal of the American Chemical Society, 136(1), 68-71 (2013-12-21)
Bis(trimethylsilylethynyl)diphenylaminoborane was reacted with the strong Lewis acid B(C6F5)3 at ambient temperature to give the borole 9 admixed with a small amount of its thermal follow-up product 12. Compound 9 was subsequently stabilized by adduct formation with pyridine (10). Treatment
He Nan et al.
Journal of chromatography. A, 1523, 316-320 (2017-06-26)
Silver ion or argentation chromatography utilizes stationary phases containing silver ions for the separation of unsaturated compounds. In this study, a mixed-ligand silver-based ionic liquid (IL) was evaluated for the first time as a gas chromatographic (GC) stationary phase for
Davide Albani et al.
Nature communications, 9(1), 2634-2634 (2018-07-08)
Ensemble control has been intensively pursued for decades to identify sustainable alternatives to the Lindlar catalyst (PdPb/CaCO3) applied for the partial hydrogenation of alkynes in industrial organic synthesis. Although the geometric and electronic requirements are known, a literature survey illustrates
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service