All Photos(1)
About This Item
Empirical Formula (Hill Notation):
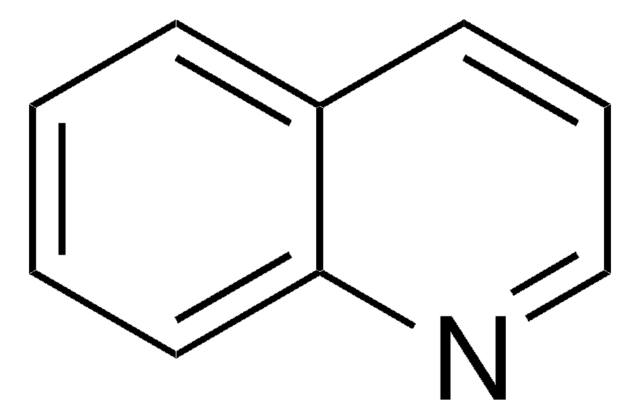
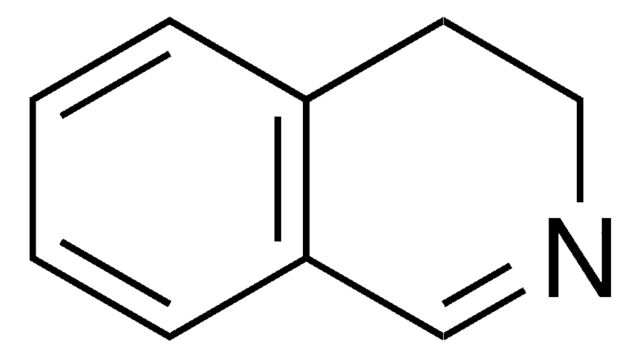
C9H11N
CAS Number:
Molecular Weight:
133.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
liquid
refractive index
n20/D 1.545 (lit.)
bp
106-108 °C/13 mmHg (lit.)
density
1.03 g/mL at 25 °C (lit.)
SMILES string
C1CCc2cnccc2C1
InChI
1S/C9H11N/c1-2-4-9-7-10-6-5-8(9)3-1/h5-7H,1-4H2
InChI key
HTMGQIXFZMZZKD-UHFFFAOYSA-N
Related Categories
General description
Reduction of 5,6,7,8-tetrahydroisoquinoline with sodium in ethanol gives trans-decahydroquinolines. 5,6,7,8-Tetrahydroisoquinoline is also referred as Bz-tetrahydroisoquinoline.
Application
5,6,7,8-Tetrahydroisoquinoline was used in total synthesis of (±)-desoxycodeine-D. It was also used in the synthesis of 7,8-dihydroisoquinolin-5(6H)-one.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
212.0 °F - closed cup
Flash Point(C)
100 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Total synthesis of (?)-desoxycodeine-d: a novel route to the morphine skeleton.
Liou J-P and Cheng C-Y.
Tetrahedron Letters, 41(6), 915-918 (2000)
A Convenient Synthesis 7, 8-Dihydroisoquinolin-5 (6H)-One.
Lardenois P, et al.
Synthetic Communications, 26(12), 2305-2308 (1996)
Preparation of cis-and trans-Decahydroisoquinolines and of Bz-Tetrahydroisoquinoline.
Witkop B.
Journal of the American Chemical Society, 70(8), 2617-2619 (1948)
Reduction of 5, 6, 7, 8-tetrahydroquinolines and 2, 3, 4, 5, 6, 7, 8, 10-octahydroquinolines to trans-decahydroquinolines.
Vierhapper FW and Eliel EL.
The Journal of Organic Chemistry, 40(19), 2734-2742 (1975)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service